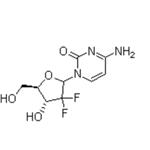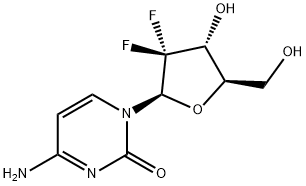
Gemcitabine
- Product NameGemcitabine
- CAS95058-81-4
- CBNumberCB4438054
- MFC9H11F2N3O4
- MW263.2
- EINECS619-100-6
- MDL NumberMFCD00869720
- MOL File95058-81-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 168,64 C |
| alpha | 365 +425.36°; D +71.51° |
| Boiling point | 482.7±55.0 °C(Predicted) |
| Density | 1.84±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | Methanol (Slightly), Water (Slightly, Heated) |
| pka | 11.65±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 95058-81-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | B76N6SBZ8R |
| ATC code | L01BC05 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H312-H315-H319-H340-H361 | |||||||||
| Precautionary statements | P280-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 21-36/38-46-62-63 | |||||||||
| Safety Statements | 25-26-36/37-53 | |||||||||
| HS Code | 29349990 | |||||||||
| Hazardous Substances Data | 95058-81-4(Hazardous Substances Data) | |||||||||
| Toxicity | LD10 i.v. in rats: 200 mg/m2 (Abbruzzese) | |||||||||
| NFPA 704: |
|