Synthesis
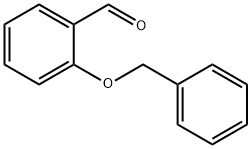
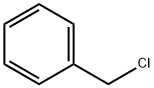
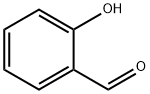
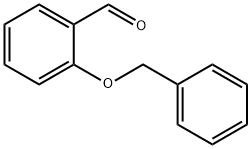
(Preparation of 2-benzyloxybenzaldehyde) 185 g of N,N-dimethylformamide (DMF) and 82.8 g (0.6 mol) of anhydrous potassium carbonate were added to a three-necked flask, and 61 g (0.5 mol) of salicylaldehyde was added slowly dropwise under stirring. Subsequently, the reaction system was warmed up to 50 °C, and 75.9 g (0.6 mol) of benzyl chloride was slowly added dropwise at this temperature for a controlled dropwise time of 1 hour. After the dropwise addition was completed, the reaction mixture was warmed up to 70 °C and the reaction was continued with stirring for 2 hours. Upon completion of the reaction, the mixture was transferred to a partition funnel and washed sequentially with 185 g of toluene and 330 g of water. The organic phases were combined and the solvent was removed by distillation under reduced pressure (internal temperature 90 °C, pressure 10 mmHg) to give 104.5 g of the brown liquid product 2-benzyloxybenzaldehyde, which was analyzed to have a purity of 97.9% and a yield of 96.5%.
References
[1] Organic and Biomolecular Chemistry, 2016, vol. 14, # 9, p. 2625 - 2636
[2] Patent: US6603029, 2003, B1
[3] Organic and Biomolecular Chemistry, 2017, vol. 15, # 17, p. 3648 - 3661
[4] Patent: CN108822095, 2018, A. Location in patent: Paragraph 0121; 0122; 0123
[5] Tetrahedron, 2015, vol. 71, # 33, p. 5217 - 5228