Antitumour Activity
Cabazitaxel is a semisynthetic taxane derivative that acts as a microtubule inhibitor. It binds to tubulin, promoting the assembly of tubulin into microtubules and inhibiting their disassembly, which results in microtubule stabilization, the inhibition of cell division, cell cycle arrest and the arrest of tumour proliferation. Cabazitaxel demonstrated antitumour activity against advanced human tumours xenografted in mice. As well as being active in docetaxel-sensitive tumours, cabazitaxel showed activity in tumour models insensitive to chemotherapy, including docetaxel. Cabazitaxel also penetrates the blood-brain barrier to a greater extent than docetaxel.
Pharmacokinetics
The pharmacokinetic data for cabazitaxel demonstrated dose proportionality, with a high plasma clearance and a long terminal half-life. The very large volume of distribution at steady state suggests extensive penetration into tissues. Of interest, cabazitaxel is able to cross the blood-brain barrier in preclinical models. Cabazitaxel is mainly metabolized by the cytochrome P450 (CYP) enzyme 3A4/5 and to a lesser extent by CYP2C8, suggesting that it has the potential to inhibit CYP3A enzymes.
Description
In June 2010, the U.S. FDA approved cabazitaxel (also referred to as
XRP6258 and RPR 116258A) in combination with the steroid prednisone
for the treatment of metastatic Castration-Resistant Prostate Cancer
(mCRPC) for patients who were previously treated with a docetaxelcontaining
regimen for late-stage disease.
Cabazitaxel is a semisynthetic analog of the
natural product taxol, which is isolated from the bark of the yew tree.
Cabazitaxel is a microtubule inhibitor that binds to the taxol-binding site of
tubulin. Similar to other tubulin inhibitors of the taxol class, cabazitaxel
inhibits microtubule disassembly resulting in mitotic blockade and cell
death. Docetaxel, also a semisynthetic taxol analog, was approved by the
FDA for the treatment of mCRPC in 2004. However, docetaxel is a substrate
for P-gp, which is thought to contribute to the constitutive and acquired
resistance of cancer cells to taxanes. Cabazitaxel has poor affinity for P-gp
and showed antitumor activity in preclinical in vitro studies and in vivo
tumor models that overexpress this protein. Cabazitaxel is synthesized on
a commercial scale from 10-deacetylbaccatin .
Chemical Properties
White solid
Originator
Sanofi-Aventis (France)
Uses
A novel semi-synthetic taxane with antitumor activity used for the treatment of castration-resistant prostate cancer. A microtubule inhibitor.
Uses
Cabazitaxel (Jevtana, XRP6258) is a semi-synthetic derivative of a natural taxoid.
Definition
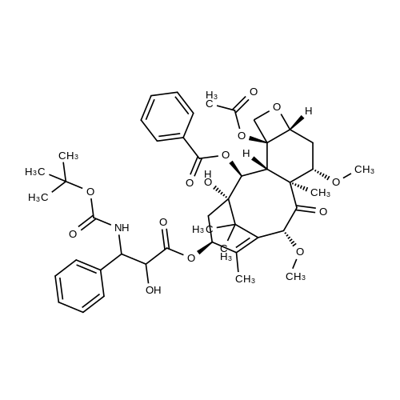
ChEBI: A tetracyclic diterpenoid that is 10-deacetylbaccatin III having O-methyl groups attached at positions 7 and 10 as well as an O-(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hy
roxy-3-phenylpropanoyl group attached at position 13. Acts as a microtubule inhibitor, binds tubulin and promotes microtubule assembly and simultaneously inhibits disassembly.
Clinical Use
Cabazitaxel was developed by Sanofi-Aventis as an intravenous injectable drug for the treatment of
hormone-refractory metastatic prostate cancer. As a microtubule inhibitor, cabazitaxel differs from docetaxel because it exhibits a much weaker affinity for P-glycoprotein (P-gp), an adenosine
triphosphate (ATP)-dependent drug efflux pump. Cancer cells that express P-gp become resistant
to taxanes, and the effectiveness of docetaxel can be limited by its high substrate affinity for P-gp.
Clinical studies confirmed that cabazitaxel retains activity in docetaxel-resistant tumors. Common
adverse events with cabazitaxel include diarrhea and neutropenia. Cabazitaxel in combination with
prednisone is an important new treatment option for men with docetaxel-refractory metastatic CRPC
(castration-resistant prostate cancer).
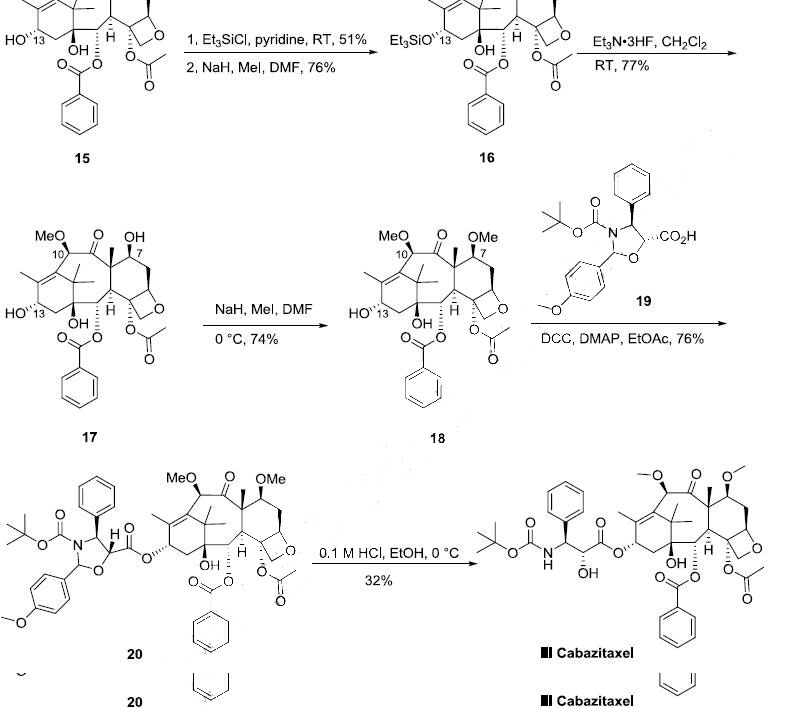
Synthesis
The semi-synthesis of cabazitaxel started from 10-
deacetylbaccatin III (15) which can be prepared from 7-xylosyl-10-deacetylbaccatin natural product
mixture according to a literature process procedure (the Scheme). 10-Deacetylbaccatin III was
protected with triethylsilyl chloride (TESCl) in pyridine to afford the corresponding 7,13-bis-silyl ether
in 51% yield, which was methylated with MeI and NaH in DMF to give 10-methoxy-7,13-bis silyl ether
16 in 76% yield. After de-silylation of 16 with triethylamine trihydrofluoride complex at room
temperature, triol 17 was obtained in 77% yield. Selective methylation of 17 with MeI and NaH in
DMF at 0 oC provided 7,10-dimethyl ether 18 in 74% yield. Compound 18 was condensed with
commercially available oxazolidinecarboxylic acid 19 in the presence of
dicyclohexylcarbodiimide/dimethylaminopyridine (DCC/DMAP) in ethyl acetate at room temperature
to generate ester 20 in 76% yield. The oxazolidine moiety of compound 20 was selectively hydrolyzed
under mild acidic conditions to yield the hydroxy Boc-amino ester derivative cabazitaxel (III) in 32%
yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: Avoid with clarithromycin, rifabutin,
rifampicin and telithromycin.
Antidepressants: Avoid with St John's wort.
Antiepileptics: Avoid with carbamazepine,
fosphenytoin, phenobarbital, phenytoin and
primidone.
Antifungals: Avoid with itraconazole, ketoconazole
and voriconazole.
Antipsychotics: Avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: Avoid with atazanavir, indinavir, ritonavir
and saquinavir.
Metabolism
Extensively metabolised in the liver (>95%), mainly by
the CYP3A4 isoenzyme (80-90%). Cabazitaxel is the
main circulating compound in human plasma. Seven
metabolites were detected in plasma (including 3 active
metabolites issued form O-demethylations), with the
main one accounting for 5% of parent exposure.
Excreted as metabolites into the urine (4%) and faeces (76%).