Pharmacological effects
Masitinib, also known as methotrexate masitinib, is studied and developed by the AB Science, a platelet-derived growth factor alpha/beta receptor tyrosine kinase inhibitor for the treatment of multiple myeloma, gastrointestinal stromal tumors and prostate cancer.
The drug had respectively, been entitled by FDA for the treatment of pancreatic cancer and ALS orphans disease in 2009 and 2015. On August 8, 2016, it has qualified as EMA orphan drug.
Masitinib is a novel kind of oral administrated tyrosine kinase inhibitors that could be targeted to immunizing important cellular mast cells and macrophages by inhibiting a certain amount of kinase. Based on its unique mechanism of action, masitinib can be developed for application in tumors, inflammatory diseases and certain central nervous system diseases.
In the oncology, due to its immunotherapy effect, Masitinib may affect the survival period of cancer patients (single administration or in combination with chemotherapy). By acting on mast cells and microglia, and further inhibiting the activation of the inflammatory process, Masitinib is effective against certain inflammatory, central nervous system disorders as well as symptoms associated with degeneration of these diseases.
Biological activity
Masitinib (AB1010) is a novel Kit and PDGFRα/β inhibitor with IC50 of 200 nM and 540 nM/800 nM, respectively, however with weak inhibits on ABL and c-Fms. Phase 3.
In vitro
Masitinib, at a concentration of ≤ 500 nM, it is a kind of ATP competitive inhibitors. Masitinib can also effectively inhibit the recombinant PDGFR and intracellular kinase Lyn, and FGFR3. However, Masitinib has a weak inhibitory effect on ABL and c-Fms. Masitinib acts on de-granulation, cytokine production, and bone marrow mast cell migration with the inhibitory effect being much stronger than imatinib. In Ba/F3 cells expressing human wild-type KIT, Masitinib can inhibit the SCF (stem cell factor)-induced cell proliferation with an IC50 being 150 nM and an IC50 value of being larger than 10 μM when inhibiting the IL-3 stimulated proliferation. In Ba/F3 cells expressing PDGFR-α, Masitinib is capable of inhibiting the PDGF-BB stimulated proliferation and PDGFR-alpha tyrosine phosphorylation with an IC50 of 300 nM. Masitinib acts on mast cell tumor cell lines and BMMC, and also inhibits the stimulated phosphorylation of human KIT tyrosine. Masitinib takes effect on the Ba/F3 cells to suppress the KIT-acquired functional mutations which include V559D mutations and Δ27 mutations with IC50s of 3 and 5 nM, respectively. Masitinib inhibits the cell proliferation of mast cell tumor cell lines including HMC-1α155 and FMA3 cells with the IC50 of 10 and 30 nM, respectively. Masitinib acts on two new ISS cell lines, inhibits cell growth and phosphorylation of PDGFR, indicating that Masitinib inhibits primary and metastatic ISS cell lines and is helpful in the clinical management of ISS.
In vivo
30 mg/kg Masitinib, when acting on the Ba/F3 transplanted tumor model expressing Δ27, is capable of inhibiting tumor growth and improving the survival time of the culture medium, and is non-toxic to both the heart and the gene. Daily oral administration of 12.5 mg/kg Masitinib improves all TTP (the grown tumor with the time). When the Masitinib and gemcitabine are used in combination to fight against the proliferation of the anti-gemcitabine cell lines during Mia Paca2 and Panc1 proliferation, they exhibit synergetic effects.
Feature
Masitinib has a better safety than other tyrosine inhibitors.
Chemical Properties
Off-White to Pale Yellow Solid
Uses
Masitinib is a novel tyrosine kinase inhibitor (TKI) being developed by AB Science to treat symptoms of amyotrophic lateral sclerosis (ALS). Masitinib controlled-release implant for treating solid neoplasm.
Definition
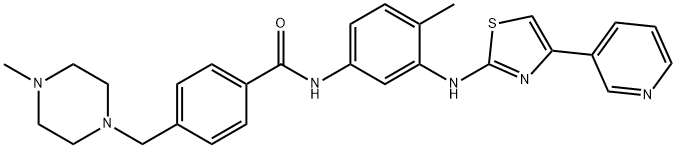
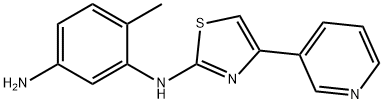
ChEBI: Masitinib is a member of the class of benzamides that is the carboxamide resulting from the formal condensation of the carboxy group of 4-[(4-methylpiperazin-1-yl)methyl]benzoic acid with the primary amino group of 4-methyl-N(3)-[4-(pyridin-3-yl)-1,3-thiazol-2-yl]benzene-1,3-diamine. It is a highly selective oral tyrosine kinase inhibitor. It has a role as a tyrosine kinase inhibitor, an antineoplastic agent and an antirheumatic drug. It is a N-alkylpiperazine, a member of 1,3-thiazoles, a member of pyridines and a member of benzamides.
Biological Activity
Masitinib is a protein tyrosine kinase inhibitor th at selectively targets stem cell factor receptor c-kit in particular and also PDGFRalpha/beta and Lyn. It is more potent and selective for c-kit than imatinib (Gleevec). Masitinib has antineoplastic and anti-inflammatory activity. It inhibits the survival, migration and activity of mast cells, key components of the inflammation response. It is used to tre at mast cell tumors in dogs and is in clinical trials for human use in rheumatoid arthritis, asthma, and as an adjuct therapy for Alzheimerμs disease.
Side effects
Masitinib is relatively well tolerated with the most common side effects being asthenia (loss of strength and/or energy), rash, nausea, oedema (fluid retention) and diarrhoea.