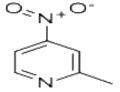
Synthesis
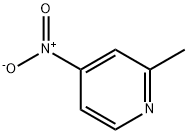
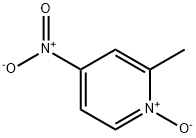
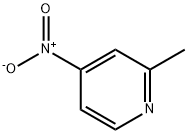
a) To a chloroform solution of 4-nitro-2-methylpyridine-N-oxide (11 g, 71.5 mmol) was slowly added a chloroform solution of phosphorus trichloride (PCl3, 33 mL, 0.37 mmol) at 0 °C and under argon protection. The reaction mixture was stirred at room temperature for 3 hours. Upon completion of the reaction, the mixture was poured into ice water and neutralized with ammonium hydroxide solution to neutral, followed by extraction with dichloromethane. The organic phases were combined and concentrated to give a yellow solid product. The solid was washed with a mixed ether-petroleum ether solvent to give 7.8 g of 2-methyl-4-nitropyridine in 79% yield with a melting point of 35 °C.
References
[1] Organic Letters, 2000, vol. 2, # 22, p. 3525 - 3526
[2] Chemical and Pharmaceutical Bulletin, 1998, vol. 46, # 10, p. 1656 - 1657
[3] Chemical and Pharmaceutical Bulletin, 1990, vol. 38, # 9, p. 2446 - 2458
[4] Journal of Organic Chemistry, 1998, vol. 63, # 15, p. 5013 - 5030
[5] Synlett, 2009, # 18, p. 2927 - 2930