Synthesis
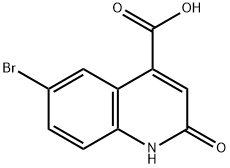
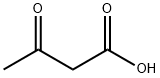
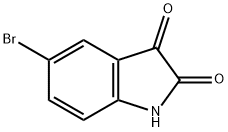
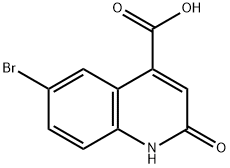
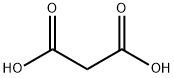
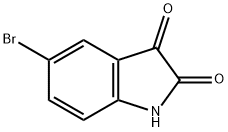
6-Bromo-2-hydroxy-4-carboxylic acid quinoline was synthesized using the method described in Scheme 6. The procedure was as follows: 5-bromoindoline-2,3-dione (0.250 g) was dissolved in tetrahydrofuran (THF, 3 ml) in a round-bottomed flask, followed by the addition of malonic acid (2 equiv). The unit was connected to a reflux condenser and heated to reflux by conventional heating and the reaction was continued overnight. After 12 hours of reaction, a solid precipitate was observed to be generated. Subsequently, the suspension was concentrated under reduced pressure to remove the solvent, and the suspension was refluxed for 4 hours after addition of an appropriate amount of water. Finally, the solid product was collected by filtration to afford 6-bromo-2-hydroxyquinoline-4-carboxylic acid (0.150 g) in solid form.