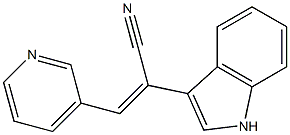
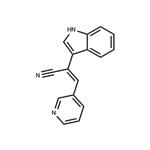
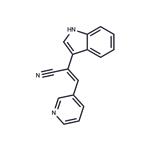
Description
Mitotic kinesin-
like protein 2 (MKLP-
2) is an N-
terminal motor from the kinesin-
6 family that is essential for normal cleavage furrow ingression and cytokinesis. Its activity mediates the relocation of the chromosome passenger proteins Aurora B kinase and survivin from the centromeres to the central spindle in anaphase cells in order to facilitate cytokinesis, avoiding the generation of binucleated cells. Paprotrain, PAssenger PROteins TRAnsport INhibitor, is the first known inhibitor of MKLP-
2. It is a cell-
permeable, reversible inhibitor uncompetitive with ATP (K
i = 3.4 μM) and noncompetitive with microtubules (K
i = 1.6 μM), demonstrating ATPase inhibition of basal and microtubule-
stimulated activities with IC
50 values of 1.35 and 0.83 μM, respectively. Paprotrain does not inhibit twelve additional kinesins, including the closely related kinesin-
6 motors MKLP-
1 and MPP1. Incubation with 10-
50 μM paprotrain results in binucleated cells, perturbing relocation of Aurora B kinase and survivin without affecting microtubule polymerization.