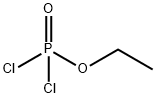
Chemical Properties
Light Yellow Liquid
Uses
Ethyl Dichlorophosphate is an intermediate used in the synthesis of b-D-2'-deoxy-2'-a-fluoro-2'-b-C-methyluridine nucleotide prodrug (PSI-7977) for treatment of hepatitis C virus. It is also used to prepare dialkylphosphocholines with antiprotozoal, antifungal and antitumor activities.
General Description
Ethyl dichlorophosphate is strongly irritating to skin. Ethyl dichlorophosphate may cause visible destruction or irreversible alterations in human skin tissue at the site of contact. Ethyl dichlorophosphate is very toxic by ingestion, inhalation, or by skin absorption. Ethyl dichlorophosphate may be combustible though Ethyl dichlorophosphate may require some effort to ignite.
Air & Water Reactions
Reacts with water to form hydrogen chloride(hydrochloric acid)
Reactivity Profile
Organophosphates, such as Ethyl dichlorophosphate, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Inhalation of vapor irritates nose and throat. Contact with liquid causes severe burns of eyes and skin. Ingestion causes severe burns of mouth and stomach.
Safety Profile
A corrosive material
that is very toxic to tissue. A severe eye,
skin, and mucous membrane irritant. When
heated to decomposition it emits very toxic
fumes of Cland POx.