Uses
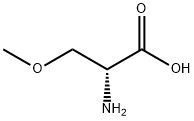
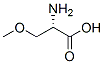
(R)-2-amino-3-methoxypropionic acid belongs to O-methyl-D-serine, is a non-natural amino acid, and is the main intermediate in the synthesis of lacoamide. It has been widely used in pharmaceutical and other industries.
Synthesis
The general procedure for the synthesis of O-methyl DL-serine from 2-amino-3-methoxypropionamide was as follows: in small-scale experiments, ACL racemase (Locus: E01594) and PepB (Locus: D84499) were added to an aqueous solution containing α-amino-β-methoxypropionamide (4). The reaction mixture (20 mL) contained 2 mM pyridoxal 5'-phosphate (PLP), 0.1 M potassium phosphate buffer (KPB, pH 9.0), and 0.5 mol/L α-amino-β-methoxypropionamide (4). Subsequently, ACL racemization enzyme (3.0 mg) and PepB (1.2 mg) were added, and the reaction was incubated for 18 h at 38°C. The progress of the reaction was monitored by periodic sampling. D/L-α-amino-β-methoxyalanamide (4) and O-methyl-L-serine (5) were determined using HPLC equipped with a Crownpak CR(+) column at a flow rate of 0.5 mL/min employing 50 mM HClO4 as a solvent system, and the absorbance of the eluate was monitored at 210 nm. Upon completion of the reaction, O-methyl-L-serine (5) was obtained by deproteinization with trichloroacetic acid and separation by Dowex-X8 (H+) column chromatography. The isolated O-methyl-L-serine (5) (7.98 mmol, 950 mg) was recrystallized from a mixed solvent of water-methanol-isopropanol-ether to give white crystals with an enol purity of >99.9%. The product has a melting point of 211-213 °C (decomposition) and a specific rotation of 1/25D (c 1, 6 mol/L HCl).1H NMR (400 MHz, D2O) data: δ 3.24 (s, 3H), 3.78-3.81 (m, 1H), 3.83 (dd, J = 3.5, 9.8 Hz, 1H), 4.01 (dd, J = 6.1, 9.8 Hz, 1H).13C NMR (100 MHz, D2O) data: δ 177.3 (CO), 76.5 (CH2), 60.2 (CH), 56.7 (OCH3). Elemental analysis (C4H9NO3) Calculated values: C, 40.32; H, 7.58; N, 11.75. Measured values: C, 40.35; H, 7.54; N, 11.77. ESI-MS (m/z): 120.1230 [M + H]+.
References
[1] Tetrahedron Asymmetry, 2012, vol. 23, # 24, p. 1653 - 1656