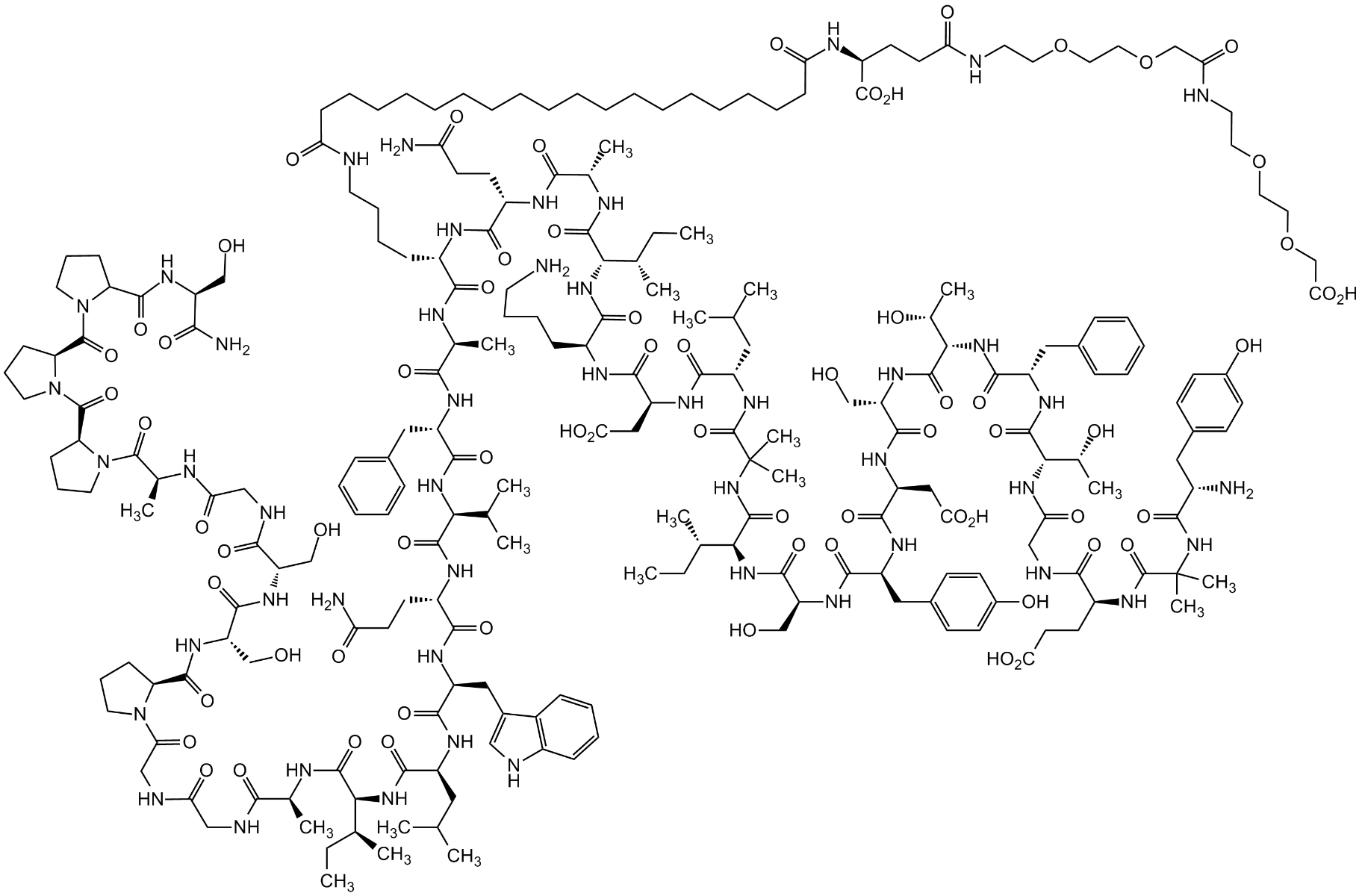
Tirzepatide
- Product NameTirzepatide
- CAS2023788-19-2
- CBNumberCB24869395
- MFC225H348N48O68
- MW4813
- EINECS200-001-8
- MOL File2023788-19-2.mol
Chemical Properties
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Solid |
| color | White to off-white |
| Sequence | Tyr-{Aib}-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-{Aib}-Leu-Asp-Lys-Ile-Ala-Gln-{diacid-C20-gamma-Glu-(AEEA)2-Lys}-Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2 |
| FDA UNII | OYN3CCI6QE |
Tirzepatide Chemical Properties,Usage,Production
Description
Tirzepatide (LY3298176) was developed as a dual agonist to both GLP-1 and gastric inhibitory polypeptide (GIP) receptors (Frias et al., 2018). Similar to GLP-1, GIP is an incretin hormone that functions to induce insulin secretion.Uses
Tirzepatide is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes. Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual function problems.Definition
Mounjaro® and Ozempic® have distinct active ingredients: tirzepatide and semaglutide, respectively. However, both of these drugs are GLP-1 agonists, which bind to GLP-1 receptors, simulate a feeling of satiety, and signal the pancreas to produce insulin.Trade name
brand name: MounjaroMechanism of action
It works to stimulate first- and second-phase insulin secretion, and reduces glucagon levels, both in a glucose-dependent manner. Tirzepatide was also shown to delay gastric emptying, lower fasting and postprandial glucose concentration, decrease food intake, 4 and reduce body weight in patients with type 2 diabetes.Pharmacology
Tirzepatide is a once-weekly GIP (glucose-dependent insulinotropic polypeptide) receptor and GLP-1 (glucagon-like peptide-1) receptor agonist that integrates the actions of both incretins into a single novel molecule. GIP is a hormone that may complement the effects of GLP-1 receptor agonists. In preclinical models, GIP has been shown to decrease food intake and increase energy expenditure therefore resulting in weight reductions, and when combined with GLP-1 receptor agonism, may result in greater effects on markers of metabolic dysregulation such as body weight, glucose and lipids. Tirzepatide is in phase 3 development for adults with obesity or overweight with weight-related comorbidity and is currently under regulatory review as a treatment for adults with type 2 diabetes. It is also being studied as a potential treatment for non-alcoholic steatohepatitis (NASH) and heart failure with preserved ejection fraction (HFpEF). Studies of tirzepatide in obstructive sleep apnea (OSA) and in morbidity/mortality in obesity are planned as well.Side effects
The overall safety and tolerability profile of tirzepatide was similar to other incretin-based therapies that have been approved for the treatment of obesity. This said, reported side effects were considerable, especially as dosage levels increased. The most common adverse events were nausea (~30%), diarrhea (~20%), constipation (~15%) and vomiting (~10%).If tirzepatide gets approved as a both a blood glucose control and anti-obesity agent, it could become a blockbuster drug. However, this isn’t a sure thing. It will have to overcome pricing and reimbursement obstacles, which have plagued similar treatments.
Synthesis
The synthesis process of tirzepatide is as follows: to a reactor was charged dichloromethane (28.6 kg), water (5.4 kg), DTT (4.3 kg), Boc-Fragment 1 + 2 + 3 + 4 (14.3 kg, 8), and TIPS (3.3 kg), resulting in a slurry. The slurry was cooled to less than 10 °C before TFA (162 kg) was added over no more than 1.75 h, resulting in a solution. The solution was warmed to 21 °C and held at this temperature for 3 h. The solution was transferred to a separate reactor, rinsing with TFA (54.3 kg). This solution was cooled to ?10 °C and charged MTBE (125.8 kg) over 2 h. Then, additional MTBE (233 kg) was charged at 17 kg/h, maintaining an internal temperature of less than ?5 °C. The resulting slurry was warmed to 0 °C and then filtered. The wet cake was washed with MTBE (2 × 11 kg/kg relative to 8) and then dried under vacuum at no more than 35 °C to obtain tirzepatide (1, 8.71 kg, 1.81 mol, 81% yield)[1].Research
Tirzepatide is in phase 3 clinical development at Eli Lilly and Company for blood glucose management in adults with type 2 diabetes, chronic weight management, and obesity-related heart failure with preserved ejection fraction. In addition, Tirzepatide is being studied as a potential treatment for non-alcoholic steatohepatitis (NASH). The molecule comprises a 39 amino acid peptide backbone and a side chain at residue 20. Of the 39 amino acids, 37 are naturally occurring (or coded), while two are noncoded aminoisobutyric acid residues at positions 2 and 13[1].Mode of action
Tirzepatide has a greater affinity to GIP receptors than to GLP-1 receptors, and this dual agonist behaviour has been shown to produce greater reductions of hyperglycemia compared to a selective GLP-1 receptor agonist. Signaling studies have shown that this is due to tirzepatide mimicking the actions of natural GIP at the GIP receptor. However, at the GLP-1 receptor, tirzepatide shows bias towards cAMP (a messenger associated with regulation of glycogen, sugar and lipid metabolism) generation, rather than β-arrestin recruitment. This combination of preference towards GIP receptor and distinct signaling properties at GLP-1 suggest this biased agonism increases insulin secretion. Tirzepatide has also been shown to increase levels of adiponectin, an adipokine involved in the regulation of both glucose and lipid metabolism, with a maximum increase of 26% from baseline after 26 weeks, at the 10 mg dosage.Clinical claims and research
Tirzepatide (Eli Lilly), a novel, once-weekly injectable dual glucose-dependent insulinotropic polypeptide (GIP) receptor and GLP-1 RA combination drug, has been developed to treat patients with T2DM. The manufacturer (Eli Lilly) announced the submission of a biologics license application with priority review to the FDA for T2DM on October 27, 2021, with a decision expected in mid-2022.References
[1] Calley, J. and W. Dhillo. “Effects of the Hormone Kisspeptin on Reproductive Hormone Release in Humans.” 2014. 0.Preparation Products And Raw materials
Tirzepatide Suppliers
Global(329)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | ||
|---|---|---|---|---|---|---|
| +8619521488211 | info@longyupharma.com | China | 2555 | 58 | ||
| +86-15377628618 +86-15377628618 |
info@yuanaotech.com | China | 6 | 58 | ||
| +86-15373193816 +86-15373193816 |
mike@ge-tian.com | China | 269 | 58 | ||
| +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12817 | 58 | ||
| +86-13129979210 +86-13129979210 |
sales@cellwh.com | China | 376 | 58 | ||
| +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 | ||
| +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 10986 | 58 | ||
| +8617709210191 | Jerry@xhobio.com | China | 882 | 58 | ||
| +86-852-57055271 +8613237129059 |
sales@rixingbiz.com | China | 232 | 58 | ||
| 15659861111 | SINOPEPT@GMAIL.COM | China | 44 | 58 |
Related articles
Is tirzepatide available for weight loss?
Tirzepatide works for weight loss by decreasing your appetite and slowing the movement of food from the stomach into the small intestine, which may make you feel full more quickly and for a longer period of time.
Mar 20,2024
View Lastest Price from Tirzepatide manufacturers
2023788-19-2, TirzepatideRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine


