Synthesis
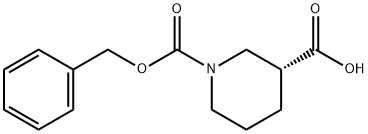
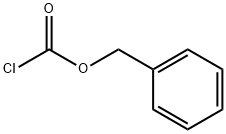
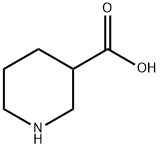
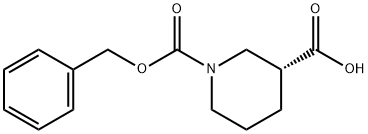
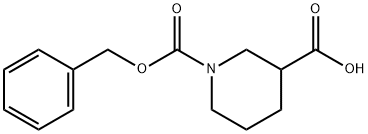
GENERAL STEPS: (R)-Piperidine-3-carboxylic acid (XXVII) (5.0 g, 39 mmol) was dissolved in a solvent mixture of 50 mL of THF and 20 mL of water and cooled to 0 °C. Subsequently, sodium bicarbonate (6.5 g, 77.4 mmol) and benzyl chloroformate (8.0 g, 46.4 mmol) were added. The reaction mixture was stirred at 0 °C for 30 min and then continued to stir at room temperature for 3 hours. After completion of the reaction, the mixture was concentrated under reduced pressure. The remaining aqueous phase was extracted with ether to remove unreacted benzyl chloroformate, then the pH was adjusted to 6 with 1 M HCl solution and extracted with ethyl acetate. The organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to give 1-benzyl ester of (R)-piperidine-1,3-dicarboxylic acid (XXVIII) (3.7 g, 36% yield). Mass spectrum (MS): m/z = 263.9 (M + 1). 1H NMR (DMSO-d6) δ = 7.38 (5H, m), 5.12 (2H, s), 4.19-3.90 (2H, m), 3.02 (1H, m), 2.44 (1H, m), 2.05 (1H, m), 1.80-1.40 (3H, m).
References
[1] Patent: WO2009/47359, 2009, A1. Location in patent: Page/Page column 160
[2] European Journal of Medicinal Chemistry, 2012, vol. 54, p. 771 - 783