Synthesis
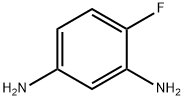
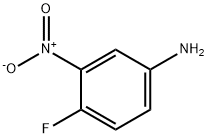
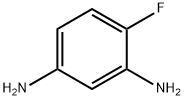
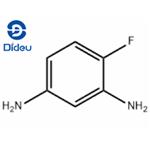
In Example 1, equimolar 4-fluoro-3-nitroaniline was substituted for m-nitroacetophenone as a raw material, and the reaction conditions were set at 80°C and 4.0 MPa for 48 hours. The rest of the operation steps were kept consistent with Example 1, and 4-fluoro-1,3-diaminobenzene was finally obtained in 94% yield. The specific operation was as follows: 16.44 mg (0.04 mmol) of silver 4,4'-dimethoxy-2,2'-bipyridine, 11.22 mg (0.1 mmol) of potassium tert-butoxide and 1 mL of 1,4-dioxane were added to an autoclave. After thorough stirring, 165.15 mg (1 mmol) of m-nitroacetophenone was added and the mixture was stirred continuously at 80 °C for 8 hours. Upon completion of the reaction, the organic phase was separated by extraction of the reaction solution using water and dichloromethane. The organic phase was subsequently dried with anhydrous Na2SO4 and purified by filtration, rotary evaporation and chromatographic separation to afford 3-acetanilide as a yellow solid in 96% yield.
References
[1] Patent: CN106748834, 2017, A. Location in patent: Paragraph 0016; 0069; 0072
[2] Patent: US4552882, 1985, A