Preparation
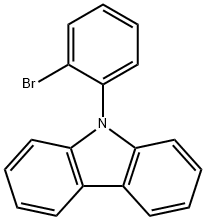
In argon atmosphere, 9-(2-bromophenyl)-9H-carbazole in THF (124 mL) was cooled in a dry ice/acetone bath, to which a 1.6 M hexane solution of n-butyllithium (17.1 mL, 27.3 mmol) was added dropwise, and the resultant solution was stirred for 2 h. After adding a solution of trimethyl borate (3.33 mL, 29.8 mmol) in THF (10 mL) dropwise, the stirring was continued for one hour, and then, the dry ice/acetone bath was removed and the temperature was raised to room temperature. The reaction liquid was cooled in an iced water bath and stirred for one hour after adding a 2 M hydrochloric acid and then the temperature was raised to room temperature. The obtained reaction liquid was extracted with ethyl acetate. The organic layer was washed with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was crystallized to obtain B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid.
![B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid](/NewsImg/2023-09-26/6383134225497416973225360.jpg)
General Description
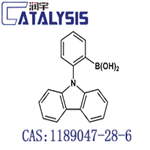
B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid, also known as 2-(9-Carbazolyl)phenylboronic acid, is a chemical compound that belongs to the carbazole family. It is a heterocyclic aromatic compound that contains a carbazole ring and a phenylboronic acid. It can be used as an intermediate in pharmaceutical synthesis.
![B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid Structure](https://www.chemicalbook.com/CAS/20150408/GIF/1189047-28-6.gif)

![B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid B-[2-(9H-Carbazol-9-yl)phenyl]boronic acid](/NewsImg/2023-09-26/6383134225497416973225360.jpg)