Synthesis
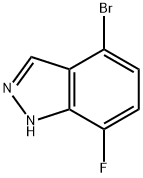
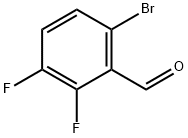
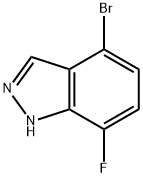
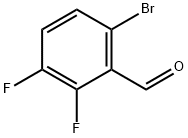
Step 2: Synthesis of 4-bromo-7-fluoro-1H-indazole; 6-bromo-2,3-difluorobenzaldehyde (11.2 g) was dissolved in dimethoxyethane (51 mL). To this solution anhydrous hydrazine (51 mL) was slowly added, followed by heating the reaction mixture to reflux for 2.5 hours. The reaction progress was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the solvent dimethoxyethane was evaporated by rotary evaporator. The remaining reaction mixture was cooled in an ice bath followed by addition of ice-water mixture. The precipitated white solid was collected by filtration and washed with cold water. The resulting solid was dissolved in dichloromethane, warmed and filtered. The filtrate was concentrated to dryness, dissolved again in dichloromethane, warmed up and filtered. 4-Bromo-7-fluoro-1H-indazole (6.19 g, white solid) was finally obtained in 32% yield. Mass spectral (MS) data: 215.0, 217.0 [M + H].