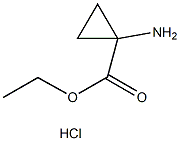
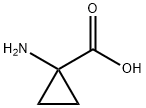
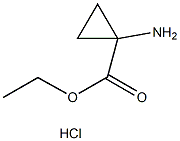
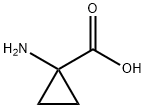
Chemical Properties
White crystalline powder
reaction suitability
reaction type: solution phase peptide synthesis
Synthesis
Thionyl chloride (150 mL, 2.056 mol) was slowly added to a suspension of 1-aminocyclopropanecarboxylic acid (100 g, 0.989 mol) in anhydrous ethanol (1 L) below 0 °C. The reaction mixture was stirred at 70 °C for 20 hours. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent: methanol, Rf = 0.4) to confirm that most of the raw materials had been consumed. Upon completion of the reaction, the solution was concentrated to give 210 g of crude product. The residue was dissolved in water and the pH was adjusted to 9-10 with potassium carbonate.Subsequently, the aqueous layer was extracted with dichloromethane (1L x 3). The organic layers were combined and concentrated to dryness. The residue was dissolved in ethyl acetate (300 mL) and a hydrochloride solution of ethyl hydrochloride (250 mL, 4 M) was slowly added at below -30°C. The mixture was stirred at 0 °C for 30 min, during which time a solid precipitated. Filtration under nitrogen protection gave ethyl 1-aminocyclopropane-1-carboxylate hydrochloride (132 g, 80.6% yield) as a white solid. The 1H-NMR data of the free amine were as follows (400 MHz, chloroform-d): δ[ppm] = 0.91-1.02 (m, 2H), 1.15-1.30 (m, 5H), 2.17 (s, 2H), 4.10 (d, 2H).
References
[1] Journal of Medicinal Chemistry, 1986, vol. 29, # 10, p. 1840 - 1846
[2] Patent: WO2014/147021, 2014, A2. Location in patent: Page/Page column 129
[3] Patent: WO2015/140195, 2015, A1. Location in patent: Page/Page column 92; 93
[4] Patent: US2006/25589, 2006, A1. Location in patent: Page/Page column 9