Uses
SB 204741 is a potent, selective SR-2B antagonist.
Definition
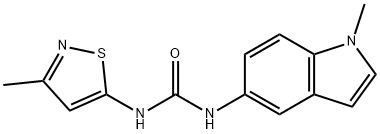
ChEBI: 1-(1-methylindol-5-yl)-3-(3-methyl-1,2-thiazol-5-yl)urea is a member of ther class of ureas that is urea in which a hydrogen attached to one of the nitrogens has been replaced by an N-methylindol-5-yl group, while a hydrogen attached to the other nitrogen has been replaced by a 3-methyl-1,2-thiazol-5-yl group. It is a potent and selective antagonist for the 5-hydroxytryptamine 2B (5-HT2B) receptor. It has a role as a receptor modulator and a serotonergic antagonist. It is a member of ureas, a member of indoles and a member of 1,2-thiazoles.
Biological Activity
Potent and selective 5-HT 2B receptor antagonist (pA 2 = 7.95). Displays ≥ 135-fold selectivity over 5-HT 2C (pK i = 5.82), 5-HT 2A (pK i < 5.2), 5-HT 1A , 1D , 1E , 5-HT 3 and 5-HT 4 receptors.
in vivo
SB-204741 (0.25~1.0 mg/kg; i.p.) induces myocardial remodeling and dose dependently improves hemodynamic and ventricular functions following isoproterenol-induced myocardial injury[1].
SB-204741 bolsters endogenous anti-oxidant enzymes activities, improves cardiac injury markers, NO level and lipid peroxidation level and attenuates TNFα level in isoproterenol-induced myocardial remodeling in rats. SB-204741 (0.5 and 1.0 mg/kg/day) pre-treatment for 28 days significantly amplifies NO level and GSH and SOD activities and attenuates TBARS level following isoproterenol-induced myocardial remodeling. SB-204741 inhibits inflammatory protein expression, upregulates autophagy and HSPs protein expressions in isoproterenol-induced myocardial remodeling in rats. SB-204741 improves myocardial architecture in isoproterenol-induced myocardial remodeling in rats[1].
| Animal Model: | Rats[1] |
| Dosage: | 0.25~1.0 mg/kg |
| Administration: | I.p. |
| Result: | Induced myocardial remodeling.
|
IC 50
human 5-HT
2B Receptor: 7.1 (pKi)