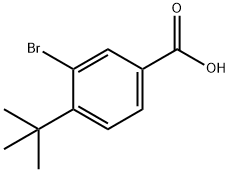
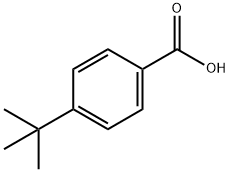
Synthesis
4-tert-butylbenzoic acid (500 mg, 2.8 mmol) was dissolved in acetic acid (11 mL), nitric acid (1.7 mL) and bromine (114 μL, 5.9 mmol) were added with rapid stirring. Subsequently, a solution of silver nitrate (1.01 g, 5.9 mmol) in water (5 mL) was slowly added to the reaction system, at which point a thick yellow precipitate was observed to form. After addition of additional acetic acid (2 mL), the reaction mixture was heated to reflux for 1 hour. Upon completion of the reaction, the reaction solution was filtered while hot and water was added dropwise to the filtrate until a white solid precipitated. The solid was collected, air dried and purified by recrystallization from acetic acid to give finally 3-bromo-4-tert-butylbenzoic acid as a white solid (263 mg, 36% yield). The product was characterized by 1H NMR (CDCl3, 300 MHz): δ 1.85 (s, 9H, (CH3)3), 7.86 (d, 1H, J = 8.5 Hz, ArH), 8.26 (dd, 1H, J = 2.0, 8.5 Hz, ArH), 8.62 (d, 1H, J = 2.0 Hz, ArH).
References
[1] Patent: WO2012/4567, 2012, A2. Location in patent: Page/Page column 91
[2] Australian Journal of Chemistry, 1990, vol. 43, # 5, p. 807 - 814
[3] Patent: WO2010/142956, 2010, A2. Location in patent: Page/Page column 28-29
[4] Journal of the Chemical Society, 1958, p. 120,126