Synthesis
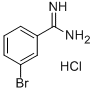
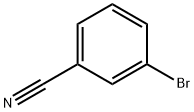
The general procedure for the synthesis of 3-bromobenzamidine hydrochloride using m-bromobenzonitrile as starting material is as follows:
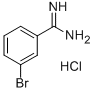
4.2.17 Synthesis of 3-bromobenzenecarboxamidine hydrochloride (23): the product was a beige solid in 81% yield.
The product characterization data were as follows:
1H NMR (400 MHz, d6-DMSO): δ 9.74 (s, 2H), 9.50 (s, 2H), 8.12 (t, J = 1.8 Hz, 1H), 7.88-7.96 (m, 2H), 7.56 (t, J = 8.1 Hz, 1H).
13C NMR (101 MHz, d6-DMSO): δ 165.0 (C), 136.9 (CH), 131.5 (CH), 131.3 (CH), 130.4 (C), 127.8 (CH), 122.4 (C).
IR (neat, ν/cm-1): 3239 (br s), 3027 (br s), 1673 (s), 1519 (s), 1465 (s), 1080 (m), 884 (m), 796 (m), 710 (s), 659 (s).
HRMS (ES-TOF +) calculated value C7H8N2Br [M+H]+: 198.9871, measured value: 198.9873.
References
[1] Bioorganic and Medicinal Chemistry, 2017, vol. 25, # 23, p. 6218 - 6223
[2] Patent: WO2003/97645, 2003, A1. Location in patent: Page/Page column 48
[3] Journal of Medicinal Chemistry, 1990, vol. 33, # 4, p. 1230 - 1241
[4] Patent: US2003/139415, 2003, A1
[5] Tetrahedron Letters, 2018, vol. 59, # 4, p. 361 - 364