Uses
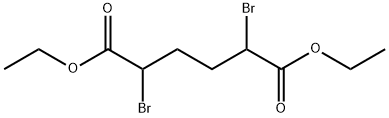
Diethyl α,α'-Dibromoadipate is an intermediate in the synthesis of Muconic Acid, a metabolite found in urine found in workers with prolonged exposure to Benzene.
Uses
Diethyl 2,5-dibromohexanedioate can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes.
Uses
Diethyl
meso-2,5-dibromoadipate may be employed as difunctional initiator during
n-butyl acrylate (
n-BuA) polymerization, leading to α,ω-bromo-poly(
n-BuA) having narrow molecular weight distribution. It was also used:
- as bifunctional atom transfer radical polymerization (ATRP) initiator
- as initiator during the solution polymerization of octadecyl ester ether dimer by ATRP
- in the synthesis of diethyl 2,5-diazidoadipate
General Description
Diethyl
meso-2,5-dibromoadipate reacts with
N-methylallylamine in the presence of potassium carbonate to yield two diastereomers of diethyl 2-allyl-
N-methylpyrrolidine-2,5-dicarboxylate.
Synthesis
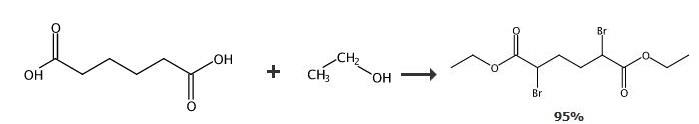
meso-2,5-Dibromo-hexanedioic acid diethyl ester JS-053: Adipic acid (795 g, 3.93 mol) was added in small portions to thionylchloride (980 mL, 5.44 mol) for 2 hours at 65 degC. This suspension was mechanically stirred and heated on an oil bath for an additional 3.5 hours at 70 degC, until the gas evolution was finished. To the UV lamp irradiated (2x 120W) solution was added dropwise bromine (664 mL, 12.48 mol) for 7 hours at 85-95 degC. After finishing of absorption of bromine, the reaction mixture was cooled down, poured into ethanol (4000 mL, 96 %) at -5 degC and mechanically stirred for an additional 1 week at rt. Crystals were filtered off and washed with ethanol (2x 300 mL, 96 %) to afford meso-2,5-dibromo-hexanedioic acid diethyl ester (JS-053, 1343 g, 3.73 mol, yield 95 %, GC 98 %, mp 66 degC/lit.