Description
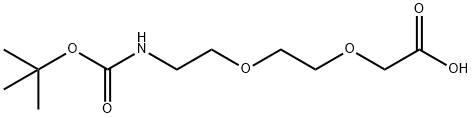
t-Boc-N-amido-PEG2-CH2CO2H is a PEG linker containing a terminal carboxylic acid and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
Uses
t-Boc-
N-amido-PEG
2-CH
2CO
2H is a heterobifunctional, PEGylated crosslinker featuring a Boc-protected amine at one end and a carboxyl group at the other. The hydrophillic PEG linker facilitates solubility in biological applications. t-Boc-
N-amido-PEG
2-CH
2CO
2H can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC
? molecules) for
targeted protein degradation.
reaction suitability
reagent type: linker
Synthesis
To a stirred solution of 2-(2-(2-Aminoethoxy)ethoxy)acetic acid (5.0 g, 30.6 mmol) in 1,4-Dioxane (50 mL), Sodium carbonate (8.12 g, 76.5 mmol, dissolved in 10 mL water) and (Boc)2O (9.98 mL, 45.7 mmol) were added and stirred at room temperature for 12 h. The progress of the reaction was monitored by TLC. The reaction mass was partitioned between diethyl ether and water. Then, the aqueous layer was made acidic (pH = 3) by 3N HC1 solution and was extracted with DCM (2 x 200 mL). The organic layer was washed with water, and brine, dried over Na2504 and evaporated under reduced pressure to yield 50 g of pure Boc-NH-PEG2-CH2COOH (Yield:62.1 per cent).