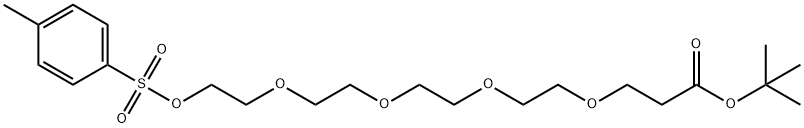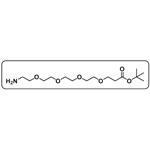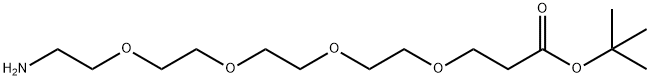
H2N-PEG4-tBu
- Product NameH2N-PEG4-tBu
- CAS581065-95-4
- CBNumberCB02546867
- MFC15H31NO6
- MW321.41
- MDL NumberMFCD11041116
- MOL File581065-95-4.mol
Chemical Properties
| Boiling point | 400.1±35.0 °C(Predicted) |
| Density | 1.040±0.06 g/cm3(Predicted) |
| refractive index | n/D 1.4508 |
| storage temp. | -20°C |
| solubility | Soluble in Water, DMSO, DCM, DMF |
| form | clear liquid |
| pka | 8.74±0.10(Predicted) |
| color | Colorless to Light yellow |
| CAS DataBase Reference | 581065-95-4 |
| UNSPSC Code | 51171641 |
| NACRES | NA.22 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| HS Code | 2922190090 | |||||||||
| NFPA 704: |
|