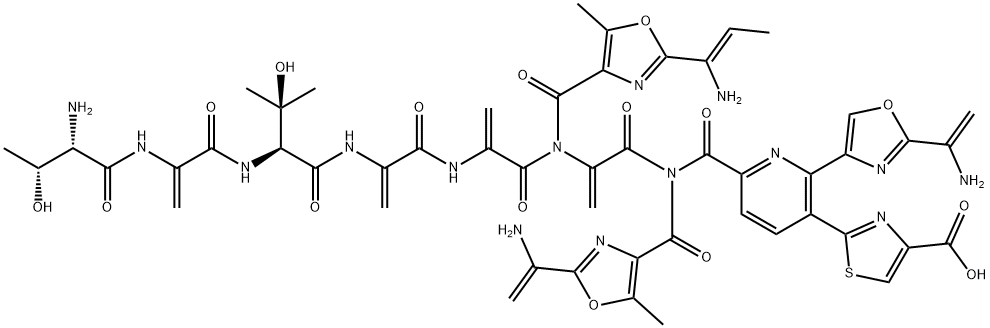
berninamycin A
- Product Nameberninamycin A
- CAS58798-97-3
- CBNumberCB01361481
- MFC51H51N15O15S
- MW1146.11
- MOL File58798-97-3.mol
- MSDS FileSDS
Chemical Properties
| Melting point | >290° |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| color | white |
| FDA UNII | M2B3X4HA2W |
berninamycin A Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Cayman Chemical 18769 | 1mg | $287 | Berninamycin A ≥99% |
Buy |
| Cayman Chemical 18769 | 5mg | $1211 | Berninamycin A ≥99% |
Buy |
| TRC B318800 | 1mg | $220 | BerninamycinA(~90%) |
Buy |
| Usbiological B1078-75 | 1mg | $523 | Berninamycin A |
Buy |
| TRC B318800 | 5mg | $970 | BerninamycinA(~90%) |
Buy |
berninamycin A Chemical Properties,Usage,Production
Uses
Berninamycin A is a cyclic thiopeptide antibiotic first isolated from S. bernensis. It inhibits protein biosynthesis in Gram positive bacteria through binding with ribosomal subunits. Cyclic thiopeptide antibiotics, including berninamycin A, induce the transcriptional activator TipA in Streptomyces.[Cayman Chemical]Uses
Berninamycin A is an antibiotic discovered in 1976 from a strain of Streptomyces. Berninamycin A is a macrocyclic "peptide" comprising atypical amino acids linked to thiazole and oxazoles. Chemically very complex, berninamycin A is an inducer of tipA, a gene that controls the bacterial transcription regulators, TipAL and TipAS, that are central to multidrug resistance. Berninamycin A is closely related to siomycin, a recently discovered inhibitor of oncogenic transcription factor, FoxM1.Biological Activity
berninamycin a is a macrocyclic thiopeptide antibiotic first isolated from s. bernensis [1]. thiopeptides are sulfur containing highly modified macrocyclic antibiotics with a central pyridine/tetrapyridine/dehydropiperidine ring with up to three thiazole substituents on positions 2, 3 and 6. thiazole antibiotics thiostrepton behaves as proteasome inhibitor in mammalian tumor cells. berninamycin showed no proteasome inhibitory activity [2]. it has been reported that the action mode of berninamycin on bacterial protein synthesis was related to that of a dissimilar compound thiostrepton. the antibiotics could bind to the complex of 23s rna with protein l11 and both affect multiple functions of the ribosomal a site [3]. berninamycin a was involved in inducing the transcriptional activator tipa in streptomyces [4].Enzyme inhibitor
This fungal antibiotic (FW = 1146.12 g/mol; CAS 58798-97-3; Soluble in DMSO), also known as Antibiotic U27810, from Streptomyces bernensis inhibits protein biosynthesis by binding to a complex formed by ribosomal 23S and the L11 protein, thereby disrupting the function of the ribosome’s A site and inhibiting protein synthesis in Gram-positive bacteria. In this respect, it has the same mode of action as thiostrepton. Streptomyces bernensis and Streptomyces azureus, which produce berninamycin and thiostrepton, respectively, possess similar ribosomal RNA methylases that mediate specific pentose-directed methylation of 23S ribosomal RNA, rendering the modified ribosomes resistant to these antibiotics.References
[1] lau r c m, rinehart k l. berninamycins b, c, and d, minor metabolites from streptomyces bernensis[j]. the journal of antibiotics, 1994, 47(12): 1466-1472.[2] pandit b, bhat u g, gartel a l. proteasome inhibitory activity of thiazole antibiotics[j]. cancer biology & therapy, 2011, 11(1): 43-47.
[3] j. thompson, e. cundliffe and m. j. r. stark. the mode of action of berninamycin and mechanism of resistance in the producing organism, streptomyces bernensis. j.gen.microbiol. 128(4), 875-884 (1982).
[4]. m. l. chiu, m. folcher, t. katoh, et al. broad spectrum thiopeptide recognition specificity of the streptomyces lividans tipal protein and its role in regulating gene expression. the journal of biological chemisty 274(29), 20578-20586 (1999).
Preparation Products And Raw materials
berninamycin A Supplier
Global(29)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| 16314854226; +16314854226 |
inquiry@bocsci.com | United States | 19741 | 58 | |
| tp@aladdinsci.com | United States | 52924 | 58 | ||
| 86-(0)29-85992781 17792393971 |
info@lynnchem.com | China | 4587 | 58 | |
| 44-20819178-90 02081917890 |
info@novachemistry.com | United Kingdom | 4381 | 58 | |
| +86-400-002-6226 +86-13028896684; |
sales@rrkchem.com | China | 57423 | 58 | |
| 18818260767 | sales@chemegen.com | China | 11218 | 58 | |
| 400-820-6829 | sales@mitachieve.com | China | 8860 | 58 | |
| 021-69568360 18916172912 |
order@med-bio.cn | China | 8140 | 58 | |
| 0519-85524369 | 3477467573@qq.com | China | 8617 | 58 | |
| 021-58000709 15900491054 |
info@scrbio.com | China | 9502 | 58 |
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine