Synthesis
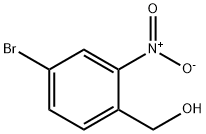
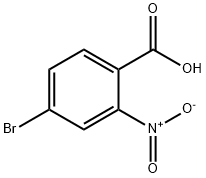
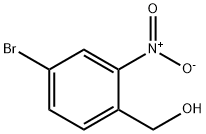
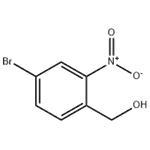
Step 1. A solution of borane tetrahydrofuran complex (1 M solution of tetrahydrofuran, 13 mL, 13 mmol) was slowly added dropwise to a tetrahydrofuran solution of 4-bromo-2-nitrobenzoic acid (2.00 g, 8.13 mmol) over 5 min at room temperature. Subsequently, the reaction mixture was heated to 72 °C and the reaction was stirred. Upon completion of the reaction, the mixture was carefully poured into saturated aqueous sodium bicarbonate solution to quench the reaction. The aqueous phase was extracted with ethyl acetate, the organic phases were combined and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give the white solid product (4-bromo-2-nitrophenyl)methanol (1.85 g, 96% yield). The product was characterized by 1H-NMR (300 MHz, CDCl3): δ 8.25 (d, J = 1.8Hz, 1H), 7.80 (dd, J = 8.1, 1.8Hz, 1H), 7.67 (d, J = 8.1Hz, 1H), 4.96 (d, J = 6.3Hz, 2H), 2.37 (t, J = 6.3Hz, 1H).
References
[1] Patent: US2007/191603, 2007, A1. Location in patent: Page/Page column 40
[2] Patent: WO2007/117607, 2007, A2. Location in patent: Page/Page column 333
[3] MedChemComm, 2018, vol. 9, # 5, p. 862 - 869
[4] Journal of Medicinal Chemistry, 2018, vol. 61, # 18, p. 8402 - 8416