Synthese
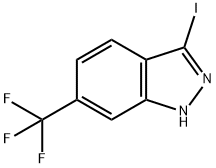
General procedure for the synthesis of 3-iodo-6-(trifluoromethyl)-1H-indazole from 6-(trifluoromethyl)-1H-indazole: At room temperature, iodine (I2, 330 mg, 1.30 mmol, 2.0 eq.) was added to a stirred solution of 6-(trifluoromethyl)-1H-indazole (121 mg, 0.65 mmol, 1.0 eq.) and potassium hydroxide (KOH 73 mg, 1.30 mmol, 2.0 eq.) in a solution of N,N-dimethylformamide (DMF, 6.5 mL). The reaction mixture was stirred for 3 h at room temperature before the reaction was quenched with saturated sodium thiosulfate (Na2S2O3, aqueous solution) and extracted with ethyl acetate (EtOAc). The organic layer was dried over anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure to give the crude product. The crude product was purified by Yamazen automated chromatography using a mixed solvent of hexane (Hex) and ethyl acetate (EtOAc) (9:1, v/v) as eluent to afford the target product, 3-iodo-6-(trifluoromethyl)-1H-indazole (169 mg, 83% yield) as a colorless solid. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3) and mass spectrometry (ESI): 1H NMR δ 10.65 (1H, broad single peak), 7.82 (1H, double peak, J = 3.0 Hz), 7.65 (1H, double peak, J = 9.0 Hz), 7.47 (1H, double-double peak, J = 9.0, 0.5 Hz); MS (ESI) m/ z 313 [(M + H)+], retention time 4.46 min (condition B).