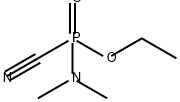
tabun
Bezeichnung:tabun
CAS-Nr77-81-6
Englisch Name:tabun
CBNumberCB51247867
SummenformelC5H11N2O2P
Molgewicht162.13
MOL-Datei77-81-6.mol
tabun physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -50° |
| Siedepunkt | bp760 240°; bp10 120°; bp9 100-108° |
| Dichte | 1.077 |
| Brechungsindex | nD20 1.4250 |
| Aggregatzustand | liquid |
| pka | -4.71±0.70(Predicted) |
| EPA chemische Informationen | Tabun (77-81-6) |
| RIDADR | 2810 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Giftige Stoffe Daten | 77-81-6(Hazardous Substances Data) |
| Toxizität | LD50 i.p. in mice: 0.6 mg/kg (Holmstedt) |
tabun Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Beschreibung
Tabun is a nonpersistent organophosphorus nerve agent that was first synthesized in 1936 by the German scientist Dr Gerhard Schrader, who would later be known as ‘the father of the nerve agents.’ Dr Schrader was developing new insecticides when he accidentally discovered tabun, the first of the nerve agents. During initial experiments testing tabun on insects, Dr. Schrader and his laboratory assistant were exposed to the vapors and quickly became ill. After learning of its properties and improving its manufacturing, the German government began synthesizing stockpiles of tabun in 1942 and had close to 13 000 tons by the end of World War II. The Allies did not become aware of tabun or other German-made nerve agents until the end of the war. Much of the German stockpiles of tabun were destroyed by dumping into the sea where it decomposes over time.
Due to their greater lethality, soman and sarin were eventually favored over tabun by the Allied nations. However, the ease of manufacturing tabun makes it a feasible option for countries that are beginning to establish a chemical weapons capability. Due in part to the simplicity and widespread knowledge about its synthesis, Iraq stockpiled and used tabun against Iranian troops in 1984 in the first confirmed instance of nerve agent use in theater. -
Chemische Eigenschaften
Liquid. Readily soluble in organic solvents; miscible with water but readily hydrolyzed; destroyed by bleaching powder, generating cyanogen chloride. Combustible. -
Chemische Eigenschaften
Tabun (GA), an organophosphorous compound, is a nerve agents, and among the most toxic of the known chemical warfare agents. Exposure to tabun can cause death in minutes. A fraction of an ounce (1-10 mL) of tabun on the skin can be fatal. GA is a clear, colorless to brownish, oily liquid, with a slight fruity odor, like almonds. No odor when pure. Warning: Odor is not a reliable indicator of the presence of toxic amounts of Tabun. It is tasteless. Tabun is chemically similar to malathion or parathion, and other organophosphates. -
Verwenden
Chemical warfare agent. -
Verwenden
Tabun is a nerve agent used in chemical warfare. It was the first of the G series of nerve agents to be described and hence was given the designation GA. -
Definition
tabun: A highly toxic colourless orbrown liquid, C5H11N2O2; r.d. 1.09;m.p. –50°C; b.p. 247.5°C. It is anorganophosphorus compound, ethylN,N-dimethylphosphoramidocyanidate.Tabun was discovered in1936 and belongs to the G-series ofnerve agents (GA). It was used byIraq in the Iran–Iraq war (1980–88). -
Allgemeine Beschreibung
Colorless to brown liquid with a faint fruity odor. Used as a chemical warfare agent. -
Air & Water Reaktionen
Hydrolysis forms hydrogen cyanide. -
Reaktivität anzeigen
When heated to decomposition, GA. emits very toxic fumes of oxides of phosphorus and nitrogen. Avoid water and acids. Can react with oxidizing materials. [EPA, 1998]. -
Hazard
Very toxic by inhalation, cholinesterase inhibitor, amilitary nerve gas, fatal dose (man) 0.01 mg/kg. -
Health Hazard
GA. is toxic by inhalation and by absorption through skin and eyes. The lethal dose for humans may be as low as 0.01 mg/kg. GA. is a nerve agent; it acts as a cholinesterase inhibitor. The median lethal dosage (respiratory) is 400 mg-minute/m3 for humans; the median incapacitating dosage is 300 mg-minute/m3. Respiratory lethal dosages kill in 1 to 10 minutes; liquid in the eye kills nearly as rapidly. Skin absorption great enough to cause death may occur in 1 to 2 minutes, but may be delayed for 1 to 2 hours. -
Health Hazard
Like soman and sarin, tabun is a highly potent cholinesterase inhibitor. It is extremely toxic by inhalation, ingestion, and absorption through the skin and eyes. The toxic effects are characteristic of deactivation of acetylcholinesterase enzyme. Small doses of tabun can produce severe poisoning, causing constriction of the pupils of the eye, respiratory distress, bronchial constriction, tremor, convulsions, and death. A fatal dose in humans may be about 0.01 mg/kg.
The LD50 value in mice from intravenous administration of tabun has been reported as 0.287 mg/kg (Tripathi and Dewey 1989). Gupta and coworkers (1987) investigated acute toxicity of tabun and its biochemical consequences in the brain of rats. An acute nonlethal dose of 200 μg/kg was injected subcutaneously. Within 0.5-1 hour, the toxicity was maximal; it persisted for 6 hours, accompanied by a sharp decline in acetylcholinesterase activity. The prolonged inhibition of this enzyme in muscle and brain may be due to storage and delayed release of tabun from nonenzymic sites. In addition, cyanide released from a tabun molecule could cause further delay in recovery from its toxic effects. Atropine and its combination with various compounds may offer protection against tabun . . -
Brandgefahr
Extremely poisonous. (Non-Specific -- Poison A, Liquid) Keep away from sparks, flames, and sources of ignition. Keep out of water sources and sewers. Hydrolysis forms hydrogen cyanide. When heated to decomposition, GA. emits very toxic fumes of oxides of phosphorus and nitrogen. Avoid water and acids. Can react with oxidizing materials. -
Sicherheitsprofil
Human poison by inhalation, skin contact, and intravenous routes. Experimental poison by ingestion, inhalation, skin contact, subcutaneous, intravenous, intraperitoneal, and intramuscular routes. A nerve gas. Vapor does not penetrate skin; Liquid does so rapidly. The primary physiological action is on the sympathetic nervous system, causing a vasoparesis ('partial paralysis of the vasomotor nerves, which control the diameter of the blood vessels). Vapors when inhaled can cause nausea, vomiting, and diarrhea, which can be followed by muscular twitclung and convulsions. Flammable when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits very toxic fumes of POx, CN-, and NOx. See also PARATHION and CYANIDE. -
mögliche Exposition
GA is a highly persistent (may remain liquid for more than 24 hours) chemical warfare agent; military nerve gas. Nerve agents are more toxic and potent than insecticides. Note: If used as a weapon, notify United States Department of Defense: Army. Damage and/or death may occur before chemical detection can take place. Use M8 paper if available (Detection: yellow) or M256-A1 Detector Kit (Detection limit: 0.005 milligram per cubic meter). -
Versand/Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard, Technical Name Required. Military driver shall be given full and complete information regarding shipment and conditions in case of emergency. AR 50-6 deals specifically with the shipment of chemical agents. Shipments of agent will be escorted in accordance with AR 740-32. -
Toxicity evaluation
Tabun and other nerve agents are irreversible cholinesterase (ChE) inhibitors. Clinical effects of exposure result primarily from inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Normally, AChE is responsible for degradation of the neurotransmitter acetylcholine, in both the peripheral and central nervous systems. Acetylcholine stimulates contraction of skeletal muscles, and hydrolysis by AChE prevents continual overstimulation of the acetylcholine receptors. Inhibition of AChE blocks degradation of acetylcholine, resulting in an accumulation of acetylcholine and cholinergic overstimulation of the target tissues. Effects of AChE inhibition include involuntary muscle contractions, seizures, and increased fluid secretion (e.g., tears and saliva). The cause of death is typically respiratory dysfunction resulting from paralysis of the respiratory muscles, bronchoconstriction, buildup of pulmonary secretions, and depression of the brain’s respiratory center.
ChE in the blood is often used to approximate AChE tissue levels following exposure to a nerve agent. Red blood cell cholinesterase (RBC-ChE) is found on erythrocytes and BuChE in blood plasma. Affinities of ChE inhibitors for BuChE or RBC-ChE vary. The turnover rate for RBC-ChE enzyme activity is the same as that for red blood cell turnover at w1% per day. Tissue AChE and plasma BuChE activities return with synthesis of new enzymes, the rate of which differs between plasma and tissues as well as between different tissues.
Binding of nerve agents to AChE is generally considered to be irreversible unless removed by therapy. Oximes are used as therapeutics to reactivate the enzyme prior to ‘aging’ or the point at which the agent–enzyme complex is covalently linked and the enzyme cannot be reactivated. Spontaneous reactivation in the absence of oximes is possible but is unlikely to occur at a rate sufficient to be clinically important. The time required for 50% of the enzyme to become resistant to reactivation varies by nerve agent. For tabun, it takes approximately 19 h for 50% of AChE to become resistant to reactivation and roughly 46 h for RBC-ChE to do so.
In addition to inhibition of AChE, nerve agents have been shown to induce noncholinergic effects, including changes in the levels of neurotransmitters other than acetylcholine. These include g-amino-butyric acid, dopamine, serotonin, and norepinephrine. While the exact mechanism by which nerve agent exposure alters the levels of these neurotransmitters is not known, it is thought that these changes may be due to a compensatory mechanism in response to overstimulation of the cholinergic system, direct action of the OP on the proteins responsible for noncholinergic neurotransmission, or perhaps both.
The pharmacologic and toxicologic effects of the nerve agents are dependent on their stability, rates of absorption by the various routes of exposure, distribution, ability to cross the blood–brain barrier, and rate of reaction. -
Inkompatibilitäten
Tabun (GA) decomposes slowly in water; hydrolysis forms hydrogen cyanide. Under acid conditions, GA hydrolyzes to form hydrofluoric acid (HF). Raising the pH increases the rate of decomposition significantly. Rapidly hydrolyzed in basic solutions (Na2CO3, NaOH, or KOH) with a half-life of 1.5 minutes at pH 11 @25℃. GA and its hydrolysis products exhibit no significant phototransformations in sunlight. Tabun and its hydrolysis products are thermally stable at temperatures less than 49 ℃. Reacts with oxidizing materials. Tabun is destroyed by bleaching powder, but the reaction produces cyanogen chloride (CNCl). Decomposes within six months @ 60 ℃. Complete decomposition in 3.5 hours @ 150℃; may produce hydrogen cyanide, oxides of nitrogen; oxides of phosphorus; carbon monoxide; and hydogen cyanide. Contact with metals may evolve flammable hydrogen gas -
Waste disposal
Principles and methods for destruction of chemical weapons: “Destruction of chemical weapons” means a process by which chemicals are converted in an essentially irreversible way to a form unsuitable for production of chemical weapons, and which in an irreversible manner renders munitions and other devices unusable as such. Each/nation/shall determine how it shall destroy chemical weapons, except that the following processes may not be used: dumping in any body of water, land burial or open-pit burning. It shall destroy chemical weapons only at specifically designated and appropriately designed and equipped facilities. Each. /nation/ shall ensure that its chemical weapons destruction facilities are constructed and operated in a manner to ensure the destruction of the chemical weapons; and that the destruction process can be verified under the provisions of this Convention. . A minimum of 56 grams of decon solution is required for each gram of GA. The decontamination solution is agitated while GA is added and the agitation is maintained for at least one hour. The resulting solution is allowed to react for 24 hours. At the end of 24 hours, the solution must be tritrated to a pH between 10 and 12. After completion of the 24 hour period, the decontamination solution must be treated with excess bleach (2.5 mole OC1-/mole GA) to destroy the CN formed during hydrolysis.Scoop up all material and place in a fully removable head drum with a high density polyethylene liner. Cover the contents with additional bleach before affixing the drum head. All contaminated clothing will be placed in a fully removable head drum with a high density polyethylene liner. Cover the contents of the drum with decontaminating solution as above before affixing the drum head. After sealing the head, the exterior of the drum shall be decontaminated and then labeled per IAW state, EPA and DOT regulations. All leaking containers shall be overpacked with vermiculite placed between the interior and exterior containers. Decontaminate and label in accordance with IAW, state, EPA, and DOT regulations. Conduct general area monitoring with an approved monitor to confirm that the atmospheric concentrations do not exceed the airborne exposure limit.
