Synthese
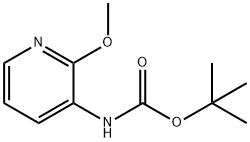
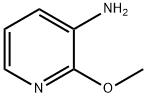
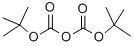
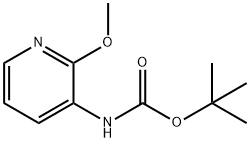
To a 2000 mL three-necked flask was added 2-methoxy-3-aminopyridine (100 g, 0.8 mol, 1.0 equiv), di-tert-butyl dicarbonate (194 g, 0.89 mol, 1.1 equiv) and 1,4-dioxane (1140 mL, 11.4 vol). The reaction mixture was heated to reflux and kept for 4 h. The progress of the reaction was monitored by thin layer chromatography (TLC) (Expander: ethyl acetate/petroleum ether = 1:4, Rf of raw material = 0.3, Rf of product = 0.55) until the raw material disappeared completely. After completion of the reaction, the reaction mixture was concentrated to afford tert-butyl 2-methoxypyridine-3-carbamate as a dark colored oil (181 g, 100% yield).1H NMR (400 MHz, DMSO-d6): δ 1.47 (s, 9H), 3.91 (s, 3H), 6.95 (m, 1H), 7.84 (m, 1H), 7.98 (d, J =8.1 Hz, 1H), 8.17 (br s, 1H).