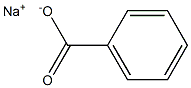
Natriumbenzoat
Bezeichnung:Natriumbenzoat
CAS-Nr532-32-1
Englisch Name:Sodium benzoate
CBNumberCB0698779
SummenformelC7H5NaO2
Molgewicht144.10317
MOL-Datei532-32-1.mol
Synonyma
Natriumbenzoat
Benzoesäure, Natriumsalz
Natriumbenzoat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | >300 °C (lit.) |
| Dichte | 1,44 g/cm3 |
| Dampfdruck | 0Pa at 20℃ |
| FEMA | 3025 | SODIUM BENZOATE |
| Flammpunkt | >100°C |
| storage temp. | room temp |
| Löslichkeit | H2O: 1 M at 20 °C, clear, colorless |
| pka | 4.03[at 20 ℃] |
| Aggregatzustand | Crystals, Granules, Flakes or Crystalline Powder |
| Farbe | White |
| PH | 7.0-8.5 (25℃, 1M in H2O) |
| Geruch (Odor) | odorless |
| Wasserlöslichkeit | soluble |
| Merck | 14,8582 |
| BRN | 3572467 |
| Stabilität | Stable, but may be moisture senstive. Incompatible with strong oxidizing agents, alkalis, mineral acids. |
| InChIKey | WXMKPNITSTVMEF-UHFFFAOYSA-M |
| LogP | 1.88 |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36/37/38-62-63-68-36 |
| S-Sätze: | 24/25-36-26 |
| WGK Germany | 1 |
| RTECS-Nr. | DH6650000 |
| TSCA | Yes |
| HS Code | 29163100 |
| Giftige Stoffe Daten | 532-32-1(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 4.07 g/kg (Smyth, Carpenter) |
Natriumbenzoat Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(1362)Suppliers
-
Telefon --
E-Mail info@radermecker-chimie.be
-
Telefon --
E-Mail marketing@harcogroup.be
-
Telefon --
E-Mail info@indis.be
-
Telefon --
E-Mail
-
Telefon --
E-Mail obe@azelis.com
-
Telefon --
E-Mail info@acros.com
1of2

