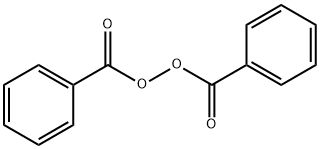
Dibenzoylperoxid
Bezeichnung:Dibenzoylperoxid
CAS-Nr94-36-0
Englisch Name:Benzoyl peroxide
CBNumberCB0484149
SummenformelC14H10O4
Molgewicht242.23
MOL-Datei94-36-0.mol
Synonyma
Dibenzoylperoxid
Benzoylsuperoxid
Dibenzoylperoxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 105 °C(lit.) |
| Siedepunkt | 176°F |
| Dichte | 1.16 g/mL at 25 °C(lit.) |
| Dampfdruck | 0.009Pa at 25℃ |
| Brechungsindex | 1.5430 (estimate) |
| Flammpunkt | >230 °F |
| storage temp. | 2-8°C |
| Löslichkeit | 0.35mg/l |
| Aggregatzustand | powder |
| Farbe | White |
| Geruch (Odor) | odorless |
| Wasserlöslichkeit | Insoluble |
| Merck | 14,1116 |
| BRN | 984320 |
| Expositionsgrenzwerte | TLV-TWA 5 mg/m3; IDLH 7000 mg/m3. |
| Stabilität | Strong oxidizer. Highly flammable. Do not grind or subject to shock or friction. Incompatible with reducing agents, acids, bases, alcohols, metals, organic materials. Contact with combustible material, heating or friction may cause fire or explosion. |
| InChIKey | OMPJBNCRMGITSC-UHFFFAOYSA-N |
| LogP | 3.2 at 20℃ |
| Kennzeichnung gefährlicher | O,Xn,N,Xi,E,T |
| R-Sätze: | 8-36/37/38-43-36-2-7-1-51/53-21/22-62-50-61-3-39/23/24/25-40 |
| S-Sätze: | 53-17-26-36/37-45-60-36/37/39-3/7-14A-14-47-35-7-61-37/39-24 |
| RIDADR | UN 3108 5.2 |
| WGK Germany | 2 |
| RTECS-Nr. | DM8575000 |
| Selbstentzündungstemperatur | 176 °F |
| TSCA | Yes |
| HazardClass | 5.2 |
| PackingGroup | II |
| HS Code | 29163200 |
| Giftige Stoffe Daten | 94-36-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 7710 mg/kg |
| IDLA | 1,500 mg/m3 |
Dibenzoylperoxid Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(517)Suppliers
-
Telefon --
E-Mail info@abcr.de
-
Telefon --
E-Mail
-
Telefon --
E-Mail @evonik.com
-
Corden Pharma International GmbH (Farchemia S.r.l )
Telefon --
E-Mail
-
United Initiators GmbH & Co. KG
Telefon --
E-Mail
-
Telefon --
E-Mail
-
Alfa Aesar, Thermo Fisher Scientific
Telefon --
E-Mail es@alfa.com
-
Telefon --
E-Mail sales@chemos-group.com
-
Telefon --
E-Mail schuchardt-vertrieb@merck.de
1of2




