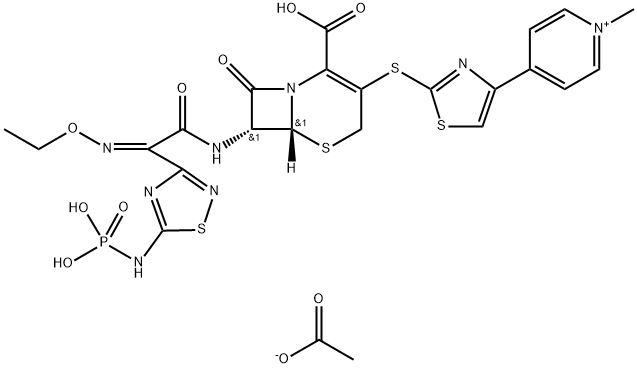
TAK 599
Bezeichnung:TAK 599
CAS-Nr400827-46-5
Englisch Name:TAK 599
CBNumberCB02628440
SummenformelC24H25N8O10PS4
Molgewicht744.72
MOL-Datei400827-46-5.mol
TAK 599 physikalisch-chemischer Eigenschaften
| storage temp. | Store at -20°C |
| Löslichkeit | DMSO : 30 mg/mL (40.28 mM; Need ultrasonic and warming) |
| Aggregatzustand | Powder |
| Farbe | White to light yellow |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)


-
Alarmwort
Achtung
-
Gefahrenhinweise
H302:Gesundheitsschädlich bei Verschlucken.
H317:Kann allergische Hautreaktionen verursachen.
H334:Kann bei Einatmen Allergie, asthmaartige Symptome oder Atembeschwerden verursachen.
-
Sicherheit
P261:Einatmen von Staub vermeiden.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P270:Bei Gebrauch nicht essen, trinken oder rauchen.
P272:Kontaminierte Arbeitskleidung nicht außerhalb des Arbeitsplatzes tragen.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P285:Bei unzureichender Belüftung Atemschutz tragen.
P301+P312:BEI VERSCHLUCKEN: Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P304+P341:BEI EINATMEN: Bei Atembeschwerden an die frische Luft bringen und in einer Position ruhigstellen, die das Atmen erleichtert
P321:Besondere Behandlung
P330:Mund ausspülen.
P333+P313:Bei Hautreizung oder -ausschlag: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P342+P311:Bei Symptomen der Atemwege: GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P363:Kontaminierte Kleidung vor erneutem Tragen waschen.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
TAK 599 Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Beschreibung
Ceftaroline fosamil, also referred to as TAK-599, is a cephalosporin antibacterial agent that was approved in the United States in October 2010 for the IV treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). Ceftaroline fosamil is the water-soluble, N-phosphono prodrug of ceftaroline (T-91825), a broad-spectrum, bactericidal agent with potent activity against methicillin-resistant Staphylococcus aureus (MRSA) strains, multidrug resistant S. pneumonia, and common gram-negative organisms. Ceftaroline binds to PBP2a as well as other PBPs with high affinity and, as a result, retains potent activity. Ceftaroline exhibits activity against most gram-positive pathogens, including β-lactam-susceptible and -resistant S. aureus, vancomycin-resistant S. aureus, and resistant and susceptible forms of S. pneumoniae but has weak activity against Enterococcus sp. The gram-negative antibacterial activity of ceftaroline is limited mainly to respiratory pathogens such as Moraxella catarrhalis and Haemophilus influenzae. -
Originator
Takeda (Japan) -
Definition
ChEBI: An acetate salt obtained by reaction of ceftaroline fosamil with one equivalent of acetic acid. A prodrug for ceftaroline, used for the treatment of adults with acute bacterial skin and skin structure infections. -
Trademarks
Teflaro -
Synthese
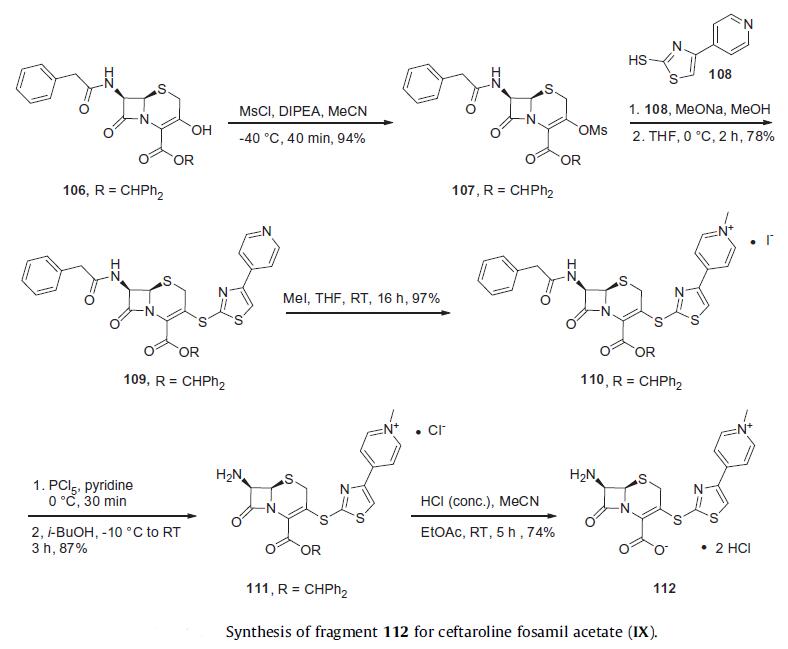
Reports from Takeda describe a process preparation of ceftaroline fosamil in 100 g scale which relies upon the assembly and union of fragments 112 and 114. The synthesis of fragment 112 began from commercially available benzhydryl 7b-[(phenylacetyl)amino]-3-hydroxy-3-cephem-4- carboxylate (106). The hydroxyl group within cephem 106 was reacted with methanesulfonyl chloride to produce mesylate 107 in 94% yield. The condensation of mesylate 107 with 4-(pyridin- 4-yl)thiazole-2-thiol 108 under the base condition of sodium methoxide gave compound 109 in 78% yield. Pyridinium salt 110 arose in quantitative yield upon subjection of 109 to iodomethane. Sequential deprotections of the amino group with phosphorous pentachloride and ester group with concentrated HCl afforded the dihydrochloride salt 112 in good yield.
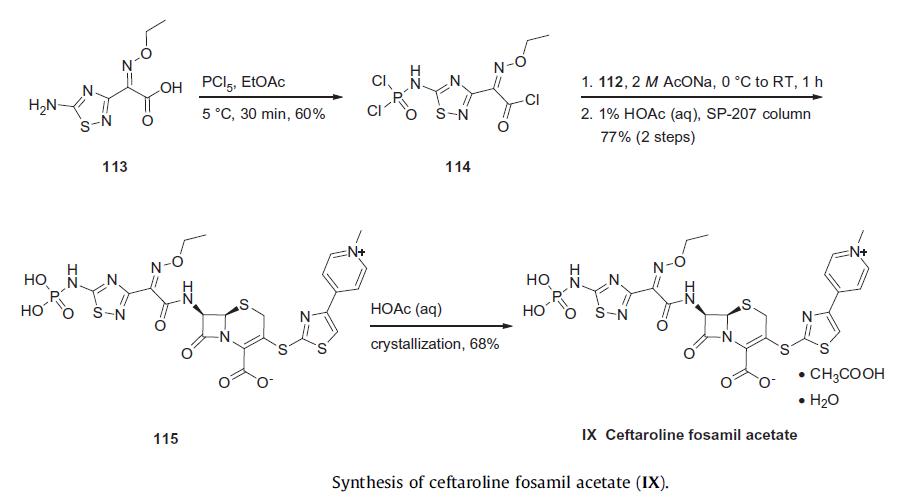
Acyl halide fragment 114 was prepared from commercially available (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-ethoxyiminoacetic acid (113) in 60% yield by dichlorophosphorylation of the amino group and concomitant acid chloride formation. Acid chloride 114 was then reacted with dihydrochloride salt 112 in the presence of sodium acetate to give N-phosphono cephem 115 in 77% yield. The crystallization of 115 in an aqueous acetic acid solution gave rise to the stable acetic acid solvate ceftaroline fosamil acetate (IX). -
Arzneimittelwechselwirkung
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be enhanced. -
Stoffwechsel
Ceftaroline fosamil (prodrug) is converted into the active ceftaroline in plasma by phosphatase enzymes. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite, ceftaroline M-1.
Ceftaroline is mainly eliminated by the kidneys. Renal clearance is approximately equal, or slightly lower than the glomerular filtration rate in the kidney, and in vitro transporter studies indicate that active secretion does not contribute to the renal elimination of ceftaroline.
TAK 599 Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(82)Suppliers
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail sales@coreychem.com
-
Hubei Jusheng Technology Co.,Ltd.
Telefon 18871490254
E-Mail linda@hubeijusheng.com
-
Telefon +86-023-6139-8061<br/>+86-86-13650506873
E-Mail sales@chemdad.com
-
Telefon +1-781-999-5354<br/>+1-00000000000
E-Mail marketing@targetmol.com
-
Wuhan Fortuna Chemical Co., Ltd
Telefon +86-027-59207850
E-Mail info@fortunachem.com
-
Telefon +1-708-310-1919<br/>+1-13798911105
E-Mail sales@invivochem.cn
-
Shandong Zhishang New Material Co., Ltd.
Telefon +8617653113209
E-Mail sales002@sdzschem.com
-
Telefon +86-852-30606658
E-Mail market18@leapchem.com
-
Hangzhou MolCore BioPharmatech Co.,Ltd.
Telefon +86-057181025280;<br/>+8617767106207
E-Mail sales@molcore.com
-
Telefon
E-Mail support@targetmol.com
1of2
