217645-70-0
基本信息
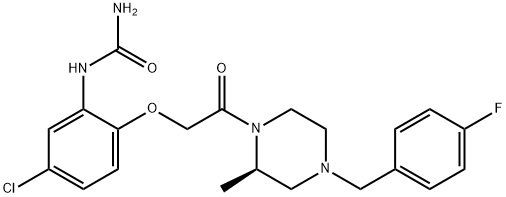
(2R)-1-[[[4-氯-2-(脲基)苯氧基]甲基]羰基]-2-甲基-4-(4-氟苄基)哌嗪
(R)-1-[5-氯-2-[2-[4-(4-氟苄基)-2-甲基哌嗪-1-基]-2-氧代乙氧基]苯基]脲
BX471(ZK-811752)
ZK811752 (BX471)
BX-471(Free base)
ZK-811752(BX-471) (free base)
BX471 - CAS 217645-70-0 - Calbiochem
ZK 811752
ZK811752
ZK-811752
BX-471
BX 471
BX-471
(5-Chloro-2-{2-[4-(4-fluoro-benzyl)-2-Methyl-piperazin-1-yl]-2-oxo-ethoxy}-phenyl)-urea
(R)-1-[5-Chloro-2-[2-[4-(4-fluorobenzyl)-2-methylpiperazin-1-yl]-2-oxoethoxy]phenyl]urea
(2R)-1-[[[4-Chloro-2-(ureido)phenoxy]methyl]carbonyl]-2-methyl-4-(4-fluorobenzyl)piperazine
物理化学性质
| 沸点 | 593.5±50.0 °C(Predicted) |
| 密度 | 1.346±0.06 g/cm3(Predicted) |
| 储存条件 | 2-8°C |
| 储存条件 | Inert atmosphere,2-8°C |
| 溶解度 | 二甲基亚砜:≥25mg/mL |
| 酸度系数(pKa) | 13.66±0.70(Predicted) |
| 形态 | 白色粉末 |
| 颜色 | 白色至棕褐色 |
| InChI | 1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 |
| InChIKey | XQYASZNUFDVMFH-CQSZACIVSA-N |
| SMILES | N(C1=CC(Cl)=CC=C1OCC(N1CCN(CC2=CC=C(F)C=C2)C[C@H]1C)=O)C(N)=O |
安全数据
| 危险性符号(GHS) |  GHS07 |
| 警示词 | 警告 |
| 危险性描述 | H302-H312-H315-H319-H332-H335 |
| 防范说明 | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 |
| 危险品标志 | Xn,N |
| 危险类别码 | 22-50 |
| 安全说明 | 61 |
| WGK Germany | 3 |
| 海关编码 | 2933.59.8000 |
| 存储类别 | 11 - Combustible Solids |
图谱信息
Urea, N-[5-chloro-2-[2-[(2R)-4-[(4-fluorophenyl)Methyl]-2-Methyl-1-piperazinyl]-2-oxoethoxy]phenyl]-(217645-70-0)核磁图(1HNMR)(R)-1-(5-氯-2-(2-(4-(4-氟苄基)-2-甲基哌嗪-1-基)-2-氧代乙氧基)苯基)脲价格(试剂级)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B6060 | BX 471 BX 471 | 217645-70-0 | 5mg | 295元 |
| 2025/12/22 | B6060 | BX 471 BX 471 | 217645-70-0 | 25mg | 1080元 |
常见问题列表
|
MIP-1α-CCR1 1 nM (Ki) |
RANTES-CCR1 2.8 nM (Ki) |
MCP-3-CCR1 5.5 nM (Ki) |
BX471 is a potent functional antagonist based on its ability to inhibit a number of CCR1-mediated effects including Ca 2+ mobilization, increase in extracellular acidification rate, CD11b expression, and leukocyte migration. BX471 demonstrats a greater than 10,000-fold selectivity for CCR1 compared with 28 G-protein-coupled receptors. BX471 is also able to displace 125 I-MIP-1α/CCL3 binding to mouse CCR1 in a concentration-dependent manner with a K i of 215±46 nM. Increasing concentrations of BX471 inhibits the Ca 2+ transients induced by MIP-1α/CCL3 in both human and mouse CCR1 with IC 50 of 5.8±1 nM and 198±7 nM, respectively. BX471 (0.1-10 μM) shows a dose-dependent inhibition of RANTES-mediated and shear-resistant adhesion on IL-1β-activated microvascular endothelium in shear flow in isolated blood monocytes. BX471 also inhibits the RANTES-mediated adhesion of T lymphocytes to activated endothelium.
BX471 (4 mg/kg, p.o. or i.v.) is orally active with a bioavailability of 60% in dogs. Furthermore, BX471 effectively reduces disease in a rat experimental allergic encephalomyelitis model of multiple sclerosis. BX471 (20 mg/kg, s.c.) reaches peak plasma levels of 9 μM by around 30 minutes, and this rapidly declines to approximately 0.4 μM after 2 hours. From 4 to 8 hours the drug plasma levels drops to 0.1 μM or lower. Mice treated with 20 mg/kg of BX471 for 10 days shows a reduction of interstitial CD45 positive leukocytes of approximately 55%. BX471 has a borderline significant effect on the number of CCR5-positive CD8 cells in the peripheral blood. BX471 reduces the amount of FSP1-positive cells by 65% in UUO kidneys as compared with vehicle control. Pretreatment witih BX471 reduces macrophage and neutrophil accumulation in kidney after ischemia-reperfusion injury.