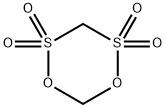
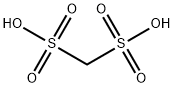
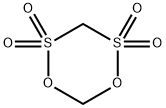
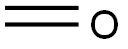Forty-eight grams (0.5 moles) of methanesulfonic acid was added to a four-necked flask equipped with a thermometer, condenser, stirrer, and a dropping funnel at room temperature and stirring was initiated. 48.5 g (0.5 mol) of sulfamic acid was slowly added dropwise, ensuring that the internal temperature of the reaction system was maintained between 25 °C and 35 °C during the dropwise addition, and the dropwise addition time was controlled to be between 3 and 4 hours. After the dropwise addition, the reaction temperature was raised to 150 °C and the reflux reaction was carried out for 3 hours. At the end of the reaction, a viscous liquid product was obtained. The product was confirmed to be methylene disulfonic acid by NMR analysis. After cooling the reaction system to room temperature, 300 g of acetic anhydride was added and stirred continuously for 0.5 h until a clarified solution was formed. In another three-necked flask, 16.5 g (0.55 mol) of formaldehyde and 123.6 g (0.9 mol) of phosphorus trichloride were added with thorough stirring to homogeneously mix the reactants, which were then heated to 120 °C and kept for 5 hours. Upon completion of the reaction, a brown liquid product was obtained. The reaction liquid was slowly poured into 100 g of ice water (temperature controlled at 0 °C to 5 °C), stirred for 0.5 h and then filtered, and the resulting solid was dried under reduced pressure at 50 °C to give 83.4 g of fine white product in 88.72% yield.