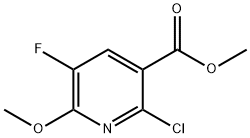
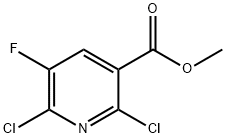
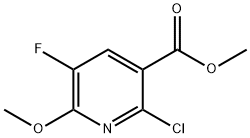
Intermediate 73b: Synthesis of methyl 2-chloro-5-fluoro-6-methoxypyridine-3-carboxylate. Sodium methanol (1.86 mL, 11.91 mmol) was added to a solution of toluene (9 mL) containing methyl 2,6-dichloro-5-fluoropyridine-3-carboxylate (1.8 g, 8.03 mmol), and the reaction mixture was stirred for 1 h at room temperature. After completion of the reaction, ethyl acetate (20 mL) and water (20 mL) were added to the mixture to separate the organic and aqueous layers. The aqueous layer was extracted with ethyl acetate (3 x 20 mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the target product methyl 2-chloro-5-fluoro-6-methoxypyridine-3-carboxylate (1.72 g, 7.83 mmol, 97% yield) as an orange solid. The product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 7.92 (1H, d, J = 9.6 Hz), 4.09 (3H, s), 3.92 (3H, s). Mass spectrometry analysis (Method 2): retention time 1.71 min, m/z 220.0 [M + H]+.