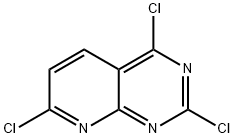
The general procedure for the synthesis of 2,4,7-trichloropyrido[2,3-d]pyrimidine (Inter.5) using 7-chloropyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (Inter.4) as a starting material is as follows: To a stirred anhydrous toluene suspension of 0.5 M Inter.4 (1 equiv.) in an inert atmosphere was slowly added diisopropylethylamine (3 equivalent). The reaction mixture was heated to 70 °C and maintained for 30 min and subsequently cooled to room temperature. Next, phosphorous oxychloride (POCl3, 3 eq.) was added and the reaction mixture was heated to 100 °C for 2.5 hours. Upon completion of the reaction, the mixture was cooled and concentrated under vacuum to give a crude product slurry. The crude product was suspended in ethyl acetate (EtOAc) and filtered through a thin diatomaceous earth pad. The filtrate was concentrated under vacuum to give a brown oil. The oily substance was dissolved in dichloromethane (CH2Cl2) and stirred on silica gel for 30 minutes. Afterwards, the silica gel was removed by filtration and the filtrate was concentrated. The crude product was purified by fast chromatography (silica gel, SiO2) to afford analytically pure 2,4,7-trichloropyrido[2,3-d]pyrimidine (48% yield, 96% purity). Mass spectrometry (LC-MS, ESP) analysis resulted in: m/z 234 [M + H]+, retention time (R/T) = 4.21 min.
![7-Chloropyrido[2,3-d]pyrimidine-2,4-diol](/CAS/GIF/938443-19-7.gif)
![2,4,7-Trichloropyrido[2,3-d]pyrimidine](/CAS/GIF/938443-20-0.gif)