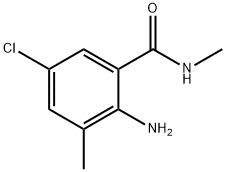
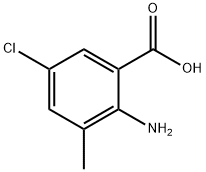
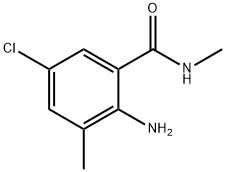
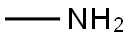First, 15 g of toluene and 50 mL of dichloromethane were sequentially added to a 200 mL autoclave. Subsequently, 0.1 g of N-hydroxyphthalimide and 0.4 g of cobalt acetylacetonate were dissolved in the above mixed solvents and stirred thoroughly. After sealing the autoclave, the air in the autoclave was replaced with oxygen for 3 times, and then pressurized to 3 MPa. The temperature of the reaction system was raised to 30 °C and kept at this temperature for 1 hour. After the reaction was completed, the mixture was cooled to room temperature and filtered to obtain a solution containing benzoic acid. The above filtrate was transferred to a 1000 mL four-necked flask equipped with a thermometer, 100 mL of glacial acetic acid was added, stirred and protected by passing nitrogen gas. The reaction temperature was raised to 40 °C, and then 30 mL of chlorine gas was added dropwise at a rate of 10 mL/min, while the temperature of the reaction solution was controlled to be 30 °C by an oil bath for 3 hours to obtain a solution containing 3,5-dichlorobenzoic acid. To this solution, 3 g of dimethylmercury was added as a shielding agent, and after stirring for 5 minutes, 10 g of Format reagent was added and the reaction was carried out for 1 hour to obtain a solution containing 3-methyl-5-chlorobenzoic acid. Under the condition of an ice bath, the temperature of the solution was controlled to be 0°C, and 5 mL of 98% concentrated sulfuric acid was added to the above solution at a rate of 5 mL/min, followed by the slow addition of 20 mL of 1 mol/L nitric acid, and the temperature of the reaction was controlled to be at 40°C. After the reaction was carried out for 1 hour, a solution containing 2-nitro-3-methyl-5-chlorobenzoic acid was obtained. The above solution was transferred to another 1000 mL four-necked flask equipped with a thermometer and 1 g of zinc flakes and 40 mL of 0.1 mol/L NaOH solution were added. The reaction temperature was controlled to be 70°C via an oil bath and the reaction was stirred for 2 hours to obtain a solution containing 2-amino-3-methyl-5-chlorobenzoic acid. At the end of the reaction, 100 mL of dichloromethane, 40 mL of N,N'-diisopropylcarbodiimide and 5 mL of 1-hydroxybenzotriazole were added to the solution and stirred for 2 hours at room temperature. Then 40 mL of methylamine was added dropwise at a rate of 10 mL/min under ice-water bath conditions, and after the dropwise addition was completed, the reaction was kept for 2 hours to obtain a solution containing 2-amino-5-chloro-N,3-dimethylbenzamide. Finally, the reaction solution was extracted with 100 mL of deionized water and 100 mL of dichloromethane, and the organic phases were combined and purified by column chromatography (eluent was a mixture of petroleum ether:ethyl acetate=1:10) to obtain a solution of the target product, 2-amino-5-chloro-N,3-dimethylbenzamide. In this method, N-hydroxyphthalimide and cobalt acetylacetonate were used as catalysts to efficiently oxidize toluene to benzoic acid under mild conditions. Meanwhile, the use of N,N'-diisopropylcarbodiimide as a condensation agent and 1-hydroxybenzotriazole as a condensation activator avoided the use of phosgene gas, simplified the synthesis steps, and significantly increased the yield to 92.6%.