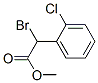
General procedure for the synthesis of α-bromo-2-chlorophenylacetic acid methyl ester from o-chlorophenylacetic acid methyl ester: N-bromosuccinimide (1.04 g, 5.84 mmol) and azobisisobutyronitrile (43 mg) were sequentially added to a stirred solution of methyl 2-chlorophenylacetate (0.86 mL, 5.31 mmol) in dichloromethane (10.2 mL, 159 mmol). The reaction mixture was stirred at 100 °C for 16 h under argon protection. After completion of the reaction, the mixture was cooled to room temperature, diluted with ether and filtered. The filtrate was concentrated under reduced pressure to remove the solvent to give an oily residue containing succinimide (456 mg), which was diluted with heptane and filtered again. Finally, the solvent was removed by distillation under reduced pressure to afford methyl α-bromo-2-chlorophenylacetate (1.38 g, 4.56 mmol) in 86% yield. The product was characterized by 1H NMR (500 MHz, CDCl3): δ 7.69 (dd, J = 7.6, 1.8 Hz, 1H), 7.31 (dd, J = 7.6, 1.7 Hz, 1H), 7.24 (td, J = 7.6, 1.7 Hz, 1H), 7.21 (dd, J = 7.5, 1.8 Hz, 1H), 5.84 (s 1H), 3.74 (s, 3H).