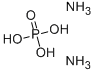
Definition
ChEBI: An inorganic phosphate, being the diammonium salt of phosphoric acid.
Potential Exposure
Used in fireproofing of textiles; wood
and paper; in soldering flux; as a fertilizer; a buffer; in
baking powder and food additives.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Seek medical attention, if necessary. Give
large quantities of water or milk. Inhalation: Move to fresh
air. Give oxygen or artificial respiration if required. Seek
medical attention, if necessary.
Shipping
There are no DOT/UN listed requirements.
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Contact with air causes
this chemical to produce anhydrous ammonia fumes.
Description
Ammonium phosphates include
mono- and diammonium orthophosphates
and ammonium polyphosphates. As discussed below, these
are made directly by reaction anhydrous ammonia with
orthophosphoric acid or superphosphoric acid. Both are
dry crystalline materials with good handling properties.
Waste Disposal
May be flushed to sewer with
huge volumes of water.
Physical properties
Colorless monoclinic crystal; saline taste; refractive index 1.52; density 1.619 g/cm3; melts at 155°C (decomposes); very soluble in water (57 g/100 g at 10°C and 106.7g/100g at 70°C, respectively); insoluble in alcohol, acetone, and liquid ammonia.
Preparation
(NH4)2HPO4 is made by reacting ammonia with phosphoric acid:
2NH3 + H3PO4 → (NH4)2HPO4.
Flammability and Explosibility
Notclassified
Agricultural Uses
With excess ammonia, the technical grade of diammonium phosphate (DAP) contains 16 to 18% nitrogen and 20to 21 % phosphorus.
DAP is an important fertilizer as well as an intermediate in the production of complex fertilizers and bulk blends.
Purification Methods
Crystallise it from water (1mL/g) between 70o and 0o. Its solubility in H2O is 59% at room temperature and 200% at the boiling point. It slowly evolves NH3 and should be stored in a well-stoppered container. After one crystallisation, ACS grade salt had Fe, Mo, Na, Se and Ti at 1, 0.2, 1.4, 0.2 and 0.8ppm, respectively. [Gmelin’s, Ammonium (8th edn) 23 pp422-426 1936.]
Toxics Screening Level
The ITSL for Diammonium hydrogen phosphate is set at the trace value of 0.1 μg/m3 as per Act 451, R232.1.(i).