Triethylene glycol (16.81 g, 112 mmol) was dissolved in 20 mL of tetrahydrofuran (THF) and preconfigured aqueous sodium hydroxide solution (6.73 g, 168 mmol, dissolved in 15 mL of water) was added. After cooling the reaction mixture to 0°C in an ice water bath, a THF solution (150 mL) of p-toluenesulfonyl chloride (TsCl, 25.67 g, 135 mmol) was added slowly and dropwise. The reaction mixture was continued to be stirred at 0°C for 1 hour. Upon completion of the reaction, the solvent was removed by rotary evaporator. The residue was dissolved in dichloromethane (DCM) and washed sequentially with saturated saline (if there is too much salt in the organic phase, it can be washed with water first). After the organic phase was dried over anhydrous magnesium sulfate, the crude product was purified by column chromatography using hexane/ethyl acetate (2:1, v/v) as eluent to afford the yellow oily target product triethylene glycol mono-p-toluenesulfonate. Yield: 89%. 1H NMR (CDCl3) δ: 2.44 (s, 3H, CH3), 3.51-3.70 (m, 12H, 6×CH2), 7.34 (d, 2H, Ar-H), 7.80 (d, 2H, Ar-H). Mass spectrum (MALDI-TOF): m/z calculated value 304.1; measured value 327.21 [M+Na]+.
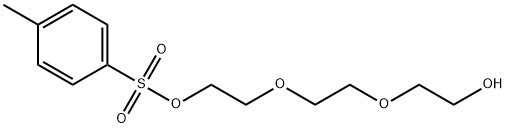

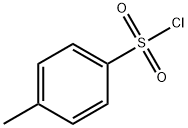
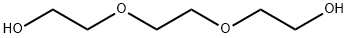
![Ethanol, 2-[2-(2-hydroxyethoxy)ethoxy]-, 1-(4-Methylbenzenesulfonate)](/CAS/GIF/77544-68-4.gif)