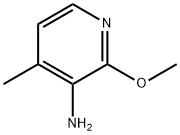
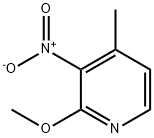
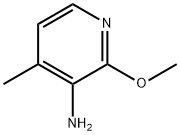
General procedure for the synthesis of 2-methoxy-3-amino-4-methylpyridine from 2-methoxy-3-nitro-4-methylpyridine:
1. a mixture of 4-methyl-2-methoxy-3-nitropyridine (4.5 g, 26.8 mmol), NH4Cl (7.16 g, 133.9 mmol) and iron powder (7.5 g, 133.9 mmol) in ethanol (50 mL) was stirred at 25°C.
2. the mixture was slowly heated to 90 °C and stirred continuously at that temperature for 2 hours.
3. upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth plug and the diatomaceous earth plug was washed with ethanol (50 mL).
4. The filtrates were combined and evaporated to dryness.
5. The residue was diluted with water (50 mL) and extracted with EtOAc (100 mL).
6. The organic layer was dried over Na2SO4 and evaporated to dryness to afford 2-methoxy-3-amino-4-methylpyridine as a solid (3.5 g, 81% yield).
Product characterization: 1H NMR (CDCl3, 400MHz) δ 7.50-7.49 (1H, d, J = 5.2Hz), 6.62-6.61 (1H, d, J = 5.2Hz), 3.97 (3H, s), 3.79-3.69 (2H, br s), 2.15 (3H, s).