General Description
White powder with a flat taste. An essential amino acid; occurs in isomeric forms.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Air & Water Reactions
Slightly soluble in water.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition this compound emits toxic fumes.
Fire Hazard
Flash point data for this chemical are not available. L-TRYPTOPHAN(73-22-3) is probably combustible.
Definition
ChEBI: The L-enantiomer of tryptophan.
Production Methods
The most attractive production processes for tryptophan are based on microorganisms used as enzyme sources or as overproducers: Enzymatic production from various precursors; Fermentative production from precursors; Direct fermentative production from carbohydrates by auxotrophic and analogue resistant regulatory mutants. L-tryptophan is synthesized from indole, pyruvate, and ammonia by the enzyme tryptophanase or from indole and L-serine/D,L-serine by tryptophan synthase these process variants were not economic due to the high costs of the starting materials. The microbial conversion of biosynthetic intermediates such as indole or anthranilic acid to L-tryptophan has also been considered as alternative for production.
However, the manufacturer using genetically modified strains derived fromBacillus amyloliquefaciens IAM 1521 was forced to stop Ltryptophan production. L-Tryptophan produced by this process was stigmatized because of side products found in the product causing a new severe disease termed eosinophilia-myalgia syndrome (EMS). One of the problematic impurities, "Peak E", was identified as 1,10-ethylidene- bis-(L-tryptophan), a product formed by condensation of one molecule acetaldehyde with two molecules of tryptophan.
Brand name
Ardeytropin;Kalma;Optimax;Sedanoct.
World Health Organization (WHO)
L-tryptophan, an essential aminoacid and precursor of serotonin,
was introduced into medicine in 1963 for the treatment of depression and sleep
disorders. Its effectiveness in these conditions has, however, never been
convincingly demonstrated. It is also widely used in dietary supplements,
parenteral nutrition preparations and dietary products for children with
phenylketonuria. In 1989, reports from the USA showed an association between the
consumption of L-tryptophan containing preparations and the development of
eosiniphilia-myalgia syndrome (EMS), a condition characterized by intense
eosinophilia, severe muscle and joint pain, swelling of the arms and legs, skin
rashes and possible fever. Some of the reported cases have been fatal. Since it is
not yet clear whether L-tryptophan itself or an unidentified contaminant is the
cause of the EMS, many drug regulatory authorities have suspended the marketing
authorization of products containing tryptophan pending further investigation,
whereas others have withdrawn these products or restricted their use.
Biochem/physiol Actions
Tryptophan (Trp) is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Increased Trp availability is necessary for the regulation of mood, cognition and behaviour. It is hypothesised that L-Trp might be useful in inducing sleep in healthy adults against the normal circadian rhythm. Trp uptake by the brain depends on the plasma ratio of Trp to all of the other LNAAs (large neutral amino acids). Higher the Trp:LNAAs ratio, greater is the Trp uptake.
storage
Store at 2-8°C, protect from light, stored under nitrogen
Purification Methods
Crystallise L-tryptophan from H2O/EtOH, wash it with anhydrous diethyl ether and dry it at room temperature in a vacuum over P2O5. It sublimes at 220-230o/0.03mm with 99% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Cox & King Org Synth Coll Vol II 612 1943, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2316-2345 1961, Beilstein 22 IV 6765.]
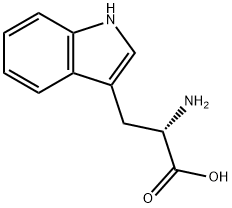
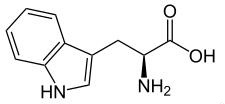 ;
;