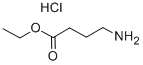
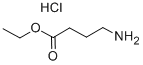
Ethyl 4-((tert-butoxycarbonyl)amino)butyrate was successfully synthesized as ethyl 4-aminobutyl ester hydrochloride (compound 44b) in 98% yield by hydrochloric acid/ethyl acetate treatment. During the reaction, stirring was continued for 3.5 h. Complete consumption of the raw material was confirmed by thin layer chromatography (TLC, unfolding agent ratio of 1:3 hexane:ethyl acetate). The product was a white solid with a melting point of 38 °C. The chemical shifts δ were analyzed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 300 MHz, MeOD) and were 4.14 (sext, 2H, J = 7.2 Hz), 3.03 (t, 2H, J = 7.2 Hz), 2.5 (t, 2H, J = 7.2 Hz), 1.99 (quint, 2H, J = 7.5 Hz), 1.25 (t, 3H, J = 7.2 Hz), respectively. J = 7.2 Hz). Nuclear magnetic resonance carbon spectroscopy (13C NMR, 75 MHz, MeOD) showed chemical shifts δ of 174.0, 61.7, 40.1, 31.8, 23.7, and 14.5, respectively. mass spectrometry (MS, CI+) analysis showed m/z of 133.11 (MD+, 54.01), 132.10 (MH+, 88.99), 86.07 ([MH+ -MeCH2O], 55.44). The results of high-resolution mass spectrometry (HRMS) analysis showed that the calculated value of C6H14NO2 (MH+, CI+) was 132.1025, and the measured value was 132.0992; the calculated value of C6H15NO2 (MH+, CI+) was 133.1103, and the measured value was 133.1098.