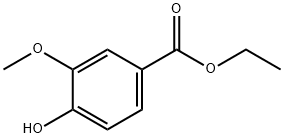
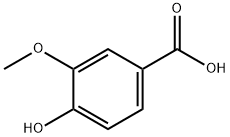
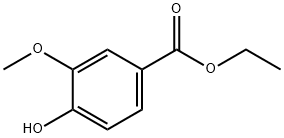
Synthesis of ethyl 4-hydroxy-3-methoxybenzoate: In a round-bottomed flask equipped with a nitrogen inlet and a magnetic stirrer, a solution of vanillic acid (10 g, 59.49 mmol) in anhydrous ethanol (400 mL) was added. Concentrated hydrochloric acid (600 mg, 6.11 mmol) was slowly added to the above solution, followed by dropwise addition of concentrated sulfuric acid. The reaction mixture was stirred under reflux conditions for 48 hours. After completion of the reaction, the solvent was removed using a rotary evaporator. Deionized water (100 mL) was added to the residue, and the green oily material was separated and discarded through a partition funnel. The resulting product was dried under vacuum to afford ethyl 4-hydroxy-3-methoxybenzoate (11.45 g, 98% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.62 (dd, J = 8.5, 2.1 Hz, 1H), 7.53 (d, J = 1.8 Hz, 1H), 6.91 (d, J = 8.6 Hz, 1H), 4.33 (q, J = 7.1 Hz, 2H), 3.91 (s, 3H), 1.36 (t, J = 7.3 Hz, 3H). Hz, 3H).HPLC-MS analysis showed the expected molecular ion peak m/z 197 (MH+) and measured m/z 197.