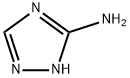
General Description
Odorless white crystals or white powder. Bitter taste. Melting point 147-159°C. Sublimes undecomposed at reduced pressure. Used as a post-emergence herbicide.
Reactivity Profile
AMITROLE(61-82-5) is a triazole derivative. The triazoles are a group that contain several derivatives that are highly explosive materials. They are sensitive to heat, friction, and impact. Sensitivity varies with the type substitution to the triazole ring. Metal chelated and halogen substitution of the triazol ring make for a particularly heat sensitive material. Azido and nitro derivatives have been employed as high explosives. No matter the derivative these materials should be treated as explosives. AMITROLE(61-82-5) forms chelates with some metals. AMITROLE(61-82-5) is corrosive to iron, copper and aluminum. Forms salts with most acids and alkalis. This compound is incompatible with strong oxidizers, strong acids, acid chlorides and acid anhydrides .
Air & Water Reactions
Water soluble. Aqueous solutions are neutral. Dust may form an explosive mixture in air.
Hazard
Toxic; carcinogen.
Potential Exposure
A potential danger to those involved
in the manufacture, formulation, and application of this
postemergence herbicide, which is now limited to noncrop
applications as a herbicide and plant growth regulator.
Some triazoles have been used as rubber components.
Fire Hazard
Literature sources indicate that this compound is non-combustible.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class:
6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous
material, Technical Name Required.
Incompatibilities
Dust may be explosive in air. Keep
away from strong oxidizers; strong acids; light and heat
(decomposes). Corrosive to iron, aluminum, and copper.
Sublimes undecomposed at reduced pressure.
Chemical Properties
Amitrole is a colorless to off-white crystalline
solid or white powder. Odorless when pure.
Chemical Properties
white powder or crystals
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.
Amitrol is resistant to hydrolysis and the action of oxidizing
agents. Burning the compound with polyethylene is
reported to result in .99% decomposition.
Uses
Herbicide; plant regulator.
Uses
Nonselective, foliage-applied, systemic, triazole herbicide used in uncropped land
and orchards to control certain grasses and to kill annual and perennial grasses and weeds.
It is also effective on poison ivy, poison oak and aquatic weeds
Production Methods
Amitrole is synthesized by condensing formic acid with
aminoguanidine and can be purified by recrystallization
from methanol.
Health Hazard
Amitrole has low acute toxicity;
in experimental animal studies subchronic
exposures were associated with changes in the
thyroid and chronic exposures were
carcinogenic.
Intentional ingestion of a mixture that contained
20 mg/kg amitrole did not cause any
signs of intoxication.1 In one reported case
study, inhalation of a large amount of amitrolecontaining
herbicide was associated with acute
toxic reaction of the lungs.2 Lung injury was
thought to be secondary to direct toxic damage
to the alveolar lining cells. The remarkable lack
of any other reports describing pulmonary toxicity
of this herbicide was noted, in addition to
the presence of other chemicals in the herbicide
solution.
Agricultural Uses
Herbicide, Plant growth regulator: A non-food use herbicide for control of grasses,
woody plants and broad-leaf weeds on hard surface and in
areas where crops are not normally grown.
Trade name
AMITRIL®; ATLAZIN®; ATLAZINE®
FLOWABLE; AT®; 3-AT®; AT-90®; ATRAFLOW PLUS®;
AZAPLANT®; AZAPLANT KOMBI®; AZOLAN®;
AZOLE®; BOROFLOW® A/ATA; CAMPAPRIM® A
1544; CDA SIMFLOW PLUS®; CHIPMAN® PATH;
CYTROLE®; DIUROL® AMITROLE; DOMATOL®;
ELMASIL®; EMISOL®; FARMCO®; HERBAZIN PLUS
SC®; HERBICIDE® TOTAL; MASCOT HIGHWAY®;
MSS AMINOTRIAZOLE®; MSS SIMAZINE®;
ORGA-414®; RADOXONE® TL; RAMIZOL®;
RASSAPRON®; SIMAZOL®; SIMFLOW PLUS®;
SOLUTION CNCENTREE T271®; SYNCHEMICALS®
TOTAL WEED KILLER; SYNTOX®; TORAPRON®;
VOROX®; WEEDAR®; WEEDAZIN®; WEEDAZOL TL
®; WEEDOCLOR®
Biochem/physiol Actions
BTK (also known as Bruton tyrosine kinase) plays a crucial role in B-lymphocyte differentiation and activation. BTK interacts with SRC homology 3 domains of FYN, LYN and HCK that are activated upon stimulation of B- and T-cell receptors.Defects in the BTK gene cause Agammaglobulinemia, an X-linked immunodeficiency characterized by failure to produce mature B lymphocyte cells and associated with a failure of Ig heavy chain rearrangement. The unique role of BTK makes it a desirable target for potential anti-cancer, anti-inflammatory and anti-viral agents as well as other treatments.
Carcinogenicity
Amitrole is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Soil. When radiolabeled amitrole-5-14C was incubated in a Hagerstown silty clay loam,
50 and 70% of the applied amount evolved as 14CO2 after 3 and 20 days, respectively. In
autoclaved soil, however, no 14CO2 was detected. The chemical degradation in soil was
probably via hydroxyl radicals (Kaufman et al., 1968). The average persistence in soils is
2–4 weeks (Hartley and Kidd, 1987)
Plant.Amitrole is transformed in plants to form the conjugate β-(3-amino-1,2,4-triazol-
1-yl)-α-alanine (Humburg et al., 1989) and/or 3-(3-amino-s-triazole-1-yl)-2-aminopropionic acid (Duke et al., 1991). Amitrole is metabolized in Canada thistl
Surface Water. In pond water, adsorption to suspended sediments was an important
process. The initial half-life was reported to be no more than 68 days. After 120 days,
20% of the applied amount remained (Grzenda et al., 1966). The biodegradation
Photolytic. Direct photolysis of amitrole is not expected to occur since the herbicide
shows little or no absorption greater than 295 nm (Gore et al., 1971)
Chemical/Physical. Reacts with acids and bases forming soluble salts (Hartley and
Kidd, 1987). Emits toxic fumes of nitrogen oxides when heated to decomposition (Sax
and Lewis, 1987); however, incineration with polyethylene results in more than 9
Purification Methods
It crystallises from EtOH (charcoal), then three times from dioxane [Williams et al. J Phys Chem 61 261 1957]. [Beilstein 26 H 137.] Possible carcinogen. [Beilstein 26 H 137, Temple & Montgomery 1,2,4-Triazoles —The Chemistry of Heterocyclic Compounds Vol 37 (Weissberger & Taylor eds.). Wiley & Sons NY 1981, ISBN 0-471-0656-6.]
References
[1] SERGEY M. DESENKO. Cyclecondensation of 3-amino-1,2,4-triazole with substituted methyl cinnamates[J]. Journal of Heterocyclic Chemistry, 2009, 36 1: 205-208. DOI:
10.1002/jhet.5570360131.
[2] XIAOTAO LIU . 3-Amino-1,2,4-triazole-derived graphitic carbon nitride for photodynamic therapy[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2021, 250: Article 119363. DOI:
10.1016/j.saa.2020.119363.