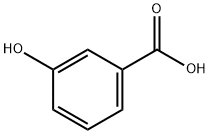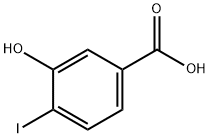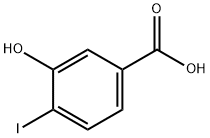
58123-77-6
| Name | 3-Hydroxy-4-iodobenzoic acid |
| CAS | 58123-77-6 |
| Molecular Formula | C7H5IO3 |
| MDL Number | MFCD02068386 |
| Molecular Weight | 264.02 |
| MOL File | 58123-77-6.mol |
Chemical Properties
| Melting point | 225-229 °C |
| Boiling point | 319.8±32.0 °C(Predicted) |
| density | 2.155±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| form | powder to crystal |
| pka | 3.90±0.10(Predicted) |
| color | White to Almost white |
| Sensitive | Light Sensitive |
| InChI | InChI=1S/C7H5IO3/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,9H,(H,10,11) |
| InChIKey | UABBBWVTEWIIMN-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C1=CC=C(I)C(O)=C1 |
Safety Data
| Hazard Codes | T,Xi |
| Risk Statements | |
| Safety Statements | |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| Hazard Note | Irritant |
| HazardClass | 6.1 |
| PackingGroup | Ⅲ |
| HS Code | 29225090 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Eye Dam. 1 Skin Irrit. 2 STOT SE 3 |