Synthesis
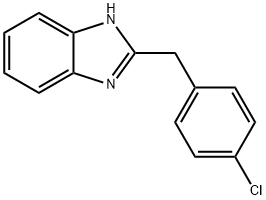
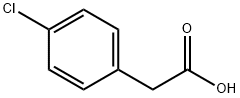
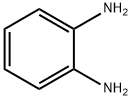
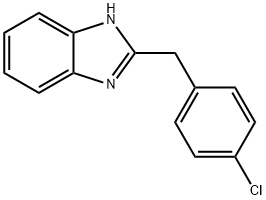
The general procedure for the synthesis of 2-(4-chlorobenzyl)benzimidazole from 4-chlorophenylacetic acid and o-phenylenediamine was as follows: 4-chlorophenylacetic acid (101.8 mmol) and sodium benzoate (0.37 mg, 0.46 mmol) were added to a 50 mL reaction flask fitted with a mechanical stirrer, a thermometer, a water separator and a reflux condenser. The reaction mixture was heated to 110 °C, followed by the addition of o-phenylenediamine (10 g, 92.5 mmol) and the temperature was raised to 120 °C and maintained for 1 hour. The course and endpoint of the reaction was monitored by thin layer chromatography (TLC) (unfolding agent: ethyl acetate to petroleum ether in a volume ratio of 1:1, Rf=0.41). Upon completion of the reaction, the reaction mixture was neutralized with 5% by weight NaOH solution to pH 7.0 to 8.5. The resulting precipitate was filtered and washed with water to neutrality to give the crude product. The crude product was recrystallized with a mixed ethanol-water solvent (1:2 to 1:3, v/v), filtered and dried to give 21.3 g of pure 2-(4-chlorobenzyl)benzimidazole.
References
[1] Patent: CN103483266, 2016, B. Location in patent: Paragraph 0054; 0055; 0056
[2] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 8, p. 1872 - 1878
[3] Journal of the American Chemical Society, 1954, vol. 76, p. 1883,1886
[4] Pharmazie, 2003, vol. 58, # 5, p. 303 - 307