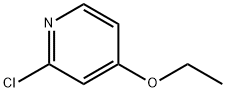
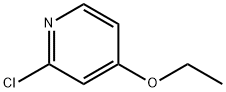
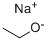GENERAL STEPS: A solution of 2-chloro-4-nitropyridine (170 g, 1070 mmol) in THF (2 L) was slowly added to sodium ethoxide (109.45 g, 1610 mmol) at 0 °C. The reaction mixture was stirred at 25°C for 12 hours. The completion of the reaction was monitored by LCMS and TLC (unfolding agent: petroleum ether/ethyl acetate = 5:1, Rf = 0.6). After completion of the reaction, the mixture was filtered and the filtrate was concentrated under reduced pressure to remove most of the solvent. The residue was extracted with ethyl acetate (800 mL x 3), the organic layer was washed with saturated sodium chloride solution (1 L), dried over anhydrous sodium sulfate, and concentrated to give 2-chloro-4-ethoxypyridine (157 g, 1.0 mol, 92% yield) as a solid.1H NMR (400 MHz, CD3OD) δ 8.15 (d, J=6.0 Hz, 1H), 6.99 (d , J=2.0Hz, 1H), 6.91-6.89 (m, 1H), 4.16-4.14 (m, 2H), 1.41-1.38 (m, 3H). es-lcms m/z 158 ([M+H]+).