5131-60-2
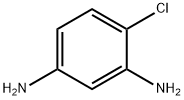
| Name | 4-Chloro-1,3-benzenediamine |
| CAS | 5131-60-2 |
| EINECS(EC#) | 225-877-9 |
| Molecular Formula | C6H7ClN2 |
| MDL Number | MFCD00025284 |
| Molecular Weight | 142.59 |
| MOL File | 5131-60-2.mol |
Chemical Properties
| Melting point | 87-90 °C (lit.) |
| Boiling point | 232.49°C (rough estimate) |
| density | 1.2124 (rough estimate) |
| refractive index | 1.5210 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| form | powder to crystal |
| pka | 3.95±0.10(Predicted) |
| color | White to Gray to Brown |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| InChI | 1S/C6H7ClN2/c7-5-2-1-4(8)3-6(5)9/h1-3H,8-9H2 |
| InChIKey | ZWUBBMDHSZDNTA-UHFFFAOYSA-N |
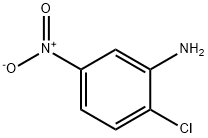
| SMILES | Nc1ccc(Cl)c(N)c1 |
| CAS DataBase Reference | 5131-60-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 27, Sup 7) 1987 |
| NIST Chemistry Reference | 1,3-Benzenediamine, 4-chloro-(5131-60-2) |
| EPA Substance Registry System | 5131-60-2(EPA Substance) |
Safety Data
| Hazard Codes | Xi |
| Risk Statements | |
| Safety Statements | |
| RIDADR | UN 1673 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | SS8800000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29215900 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
| Safety Profile | |
| Hazardous Substances Data | 5131-60-2(Hazardous Substances Data) |