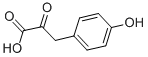
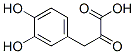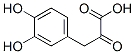General procedure for the synthesis of 3-(3,4-dihydroxyphenyl)-2-oxopropanoic acid from 4-hydroxyphenylpyruvic acid: the reaction was carried out under homogeneous and non-homogeneous conditions in a CH2Cl2/buffer system. This was done as follows: phenol (0.05 mmol), tyrosinase (600 IU) and an appropriate amount of 0.1 M sodium phosphate buffer (pH 7, CH2Cl2/buffer volumetric ratio of about 1:0.1) were suspended in CH2Cl2 (2.5 mL) and preheated at 50 °C. Subsequently, the reaction system was transferred to a 25 °C environment with continuous stirring under O2 atmosphere for 24 hours. The reaction process was monitored by thin layer chromatography (TLC, unfolding agent was hexane/EtOAc=2.0:1.0). After complete conversion of the substrate, the immobilized enzyme was recovered by filtration, and the organic layer was separated and concentrated under reduced pressure. The crude product was solubilized with equal volumes of H2O and THF (2.0 mL), Na2S2O4 was added and stirred for 5 min. The reaction mixture was diluted with EtOAc (4.0 mL) and the aqueous phase was separated from the organic phase. The organic phase was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude product was purified by fast column chromatography if necessary. The structure of the products was confirmed by 1H NMR, 13C NMR, GC-MS (after methylsilylation), and elemental analysis.The GC-MS analysis was done on a Shimadzu GC-MS QP5050 instrument with an SPB column (25 m×0.25 mm, film thickness 0.25 μm), and the initial temperature was maintained at 100 °C for 2 min, and the initial temperature was then increased to 2 μm at a rate of 10 °C/ The initial temperature of 100 °C was held for 2 min, and then the temperature was increased to 280 °C at a rate of 10 °C/min and held for 25 min. The inlet temperature was set at 280 °C. High purity helium was used as the carrier gas at a flow rate of 2.7 mL/min. The mass spectrometry acquisition was performed in 70 eV electron bombardment ionization mode. Dodecane was used as an internal standard for quantitative analysis. The MS data corresponded to fully methylsilylated derivatives except for compounds 3c and 3d.