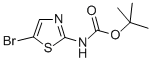
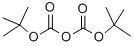
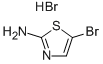
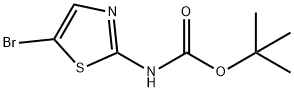
General procedure for the synthesis of N-BOC-2-amino-5-bromothiazole from di-tert-butyl dicarbonate and 2-amino-5-bromothiazole hydrobromide: di-tert-butyl dicarbonate [(Boc)2O, 100.7 g, 0.461 mol, 1.2 eq.] was added to 900 mL of THF and 135 mL of pyridine (DMAP, 1.18 g, 9.7 mmol, 0.025 eq.) containing 2-amino-5-bromothiazole monohydrobromide (100 g, 0.385 mol, 1.0 eq.) and 4-(dimethylamino)pyridine (DMAP, 1.18 g, 9.7 mmol, 0.025 eq.) in a mixture of 900 mL THF and 135 mL Et3N. The reaction mixture was cooled to 0 °C using an ice bath. Subsequently, the reaction mixture was stirred at room temperature overnight, followed by vacuum concentration. The residue was stirred in EtOAc/heptane (1:10, 250 mL) at room temperature overnight and then filtered. The filtrate was washed with brine, dried, filtered, and concentrated in vacuo to afford the intermediate N-BOC-2-amino-5-bromothiazole as a yellow solid (91% yield).