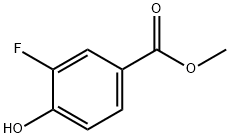
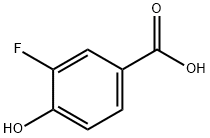
Procedure for the synthesis of methyl 3-fluoro-4-hydroxybenzoate (T10.1): 3-fluoro-4-hydroxybenzoic acid (5.03 g, 32.22 mmol, commercially available from Sigma-Aldrich, St. Louis, MO, USA), methanol (50.0 mL), and cold sulfuric acid (2.0 mL) were added to a round bottom flask. The reaction mixture was heated to 80 °C and the progress of the reaction was monitored by thin layer chromatography (TLC). After 20.5 hours of reaction, the solvent was removed by distillation under reduced pressure. The residue was diluted with ether and the organic phase was sequentially washed twice with saturated aqueous sodium bicarbonate and once with brine, followed by drying with anhydrous sodium sulfate. After filtration, the organic solvent was removed by evaporation under reduced pressure to give the product T10.1 (4.79 g, 87% yield) as a white solid. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.81 (2H, m), 7.06 (1H, t, J = 8.4 Hz), 5.62 (1H, d, J = 4.3 Hz), 3.91 (3H, s).