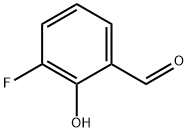
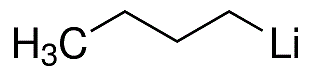
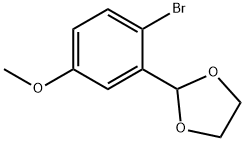
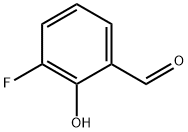
A hexane solution of n-butyllithium (17.07 mL, 1.42 M, 25.1 mmol) was slowly added dropwise to a stirred solution of 2-(2-bromo-5-methoxyphenyl)-1,3-dioxolane (5.00 g, 19.3 mmol) dissolved in dry tetrahydrofuran (60 mL) at -78°C. The reaction mixture was stirred continuously at -78 °C for 30 min before being quenched by the addition of iodomethane (2.40 mL, 38.6 mmol). After quenching, the solution was continued stirring at -78 °C for 20 min, followed by termination of the reaction with saturated aqueous ammonium chloride solution (60 mL). The organic phase was separated, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by fast column chromatography using hexane:ethyl acetate (10:1) as eluent to afford the light yellow oily target product 3-fluoro-2-hydroxybenzaldehyde (3.07 g, 82% yield). Thin layer chromatography showed an Rf value of 0.40 (Expanding agent: hexane:ethyl acetate, 10:1). The NMR hydrogen spectrum (300 MHz, CDCl3) data were as follows: δ 7.20-6.99 (2H, m, Ar-H), 6.75 (1H, dd, Ar-H), 5.92 (1H, s, CH), 4.20-4.01 (4H, m, 2 × CH2), 3.80 (3H, s, OCH3), 2.32 (3H, s, CH3) .